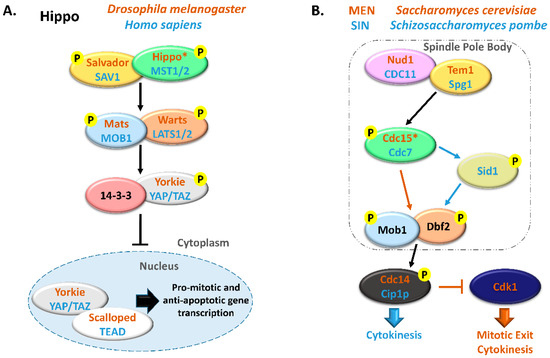
2. MOB Proteins in Multicellular Eukaryotes
The MOB family proteins are currently classified into four isotypes that tend to be functionally different within each species: MOB1, MOB2, MOB3, and MOB4/Phocein. Some species, particularly more complex organisms, also present sub-isotypes that tend to be functionally similar or redundant within each species, e.g., MOB1A and MOB1B. In several publications MOB proteins have been referred as MOB3/Phocein [
9,
22,
23,
24,
25], however this nomenclature has been reviewed based on
Homo sapiens designations (described by Gundogdu et al., 2019) [
18] and during this revision the researchers will refer to these proteins using the updated designation of MOB4/Phocein.
2.1. Functions of MOB Proteins
2.1.1. Tissue Homeostasis: MOB as Regulators of Cell Proliferation and Apoptosis
MOB proteins are tumor suppressors playing a critical role in regulating tissue homeostasis maintenance. In
D. melanogaster, it was shown that DmMOB1 (Mats) controls cell proliferation and apoptosis through interaction with DmLATS (Warts), and its lethal depletion phenotype is rescued by HsMOB1 showing function conservation from invertebrates to vertebrates [
2,
26]. Indeed,
H. sapiens, HsMOB1 also presents tumor suppressor activity, by phosphorylating HsLATS1, which can be triggered by HsMST1/2 phosphorylation but this is not essential (
Figure 2 and
Table 1) [
27,
28]. HsMOB1 tumor suppressor activity involves apoptotic signaling through Hippo pathway activation [
29]. DmMOB1 tumor suppressor activity independent of DmMST (Hippo) phosphorylation also occurs in
D. melanogaster [
26]. Both HsMOB1 and HsMOB2 have been implicated in tissue growth suppression in cancer development [
30,
31,
32,
33]. Conversely, Chen et al. (2018) [
34] showed that the cancer promoter complex HsMST4-HsMOB4/Phocein negatively regulates the tumor-suppressing complex HsMST1-HsMOB1 in pancreatic cancer. HsMOB4/Phocein and HsMST4 integrate the Striatin-interacting phosphatase and kinase (STRIPAK) complex [
35]. The STRIPAK complex also includes the protein phosphatase PP2A and regulates vesicular trafficking, microtubule cytoskeleton and morphogenesis [
9]. HsMOB3 also shows tumorigenic properties in glioblastoma cells by suppressing HsMST1 activity [
36]. In
Mus musculus, MmMOB1 functions as a tumor suppressor and tissue homeostasis factor as a member of Hippo signaling, namely by controlling apoptotic signaling in keratinocytes [
10,
37,
38,
39]. MmMOB1 also participates in renal homeostasis, MmMOB1 mediated Hippo activation, through MmLATS1 and MmYAP phosphorylation, is associated with diminished renal fibrosis [
40]. The Wnt (wingless integrated) pathway is also activated but seems to have an opposite association. In
Canis familiaris, CfMOB1-CfLATS1 Hippo signaling appears to regulate photoreceptor homeostasis [
41]. In
Gallus gallus, GgMOB2 interacts with GgSAV1 which acts as a growth suppressor through Hippo signaling [
42].
Arabidopsis thaliana AtMOB1 regulates plant growth and development, and tissue homeostasis through interaction with AtMST, known as SIK1 [
43,
44,
45,
46]. In other angiosperms,
Medicago sativa, MsMOB1 also regulates cell proliferation [
47]. However, Ms
Mob1 does not complement budding yeast MOB1 temperature sensitive growth phenotype. In the fungus
Neurospora crassa, Nc
Mob1 gene deletion results in overall reduced mycelium growth [
3]. Nc
Mob2 gene deletion exhibited phenotypes similar to Nc
Mob1 but with less intensity. Nc
Mob4 gene deletion resulted in a very mild decrease in tissue growth.
Aspergillus nidulans AnMOB4/Phocein, which integrates the STRIPAK complex, also showed tumor suppressor properties [
22]. Overall, several MOB isotypes are involved in the regulation of cell proliferation and apoptosis and its activation induces inhibition or promotion of tissue growth.
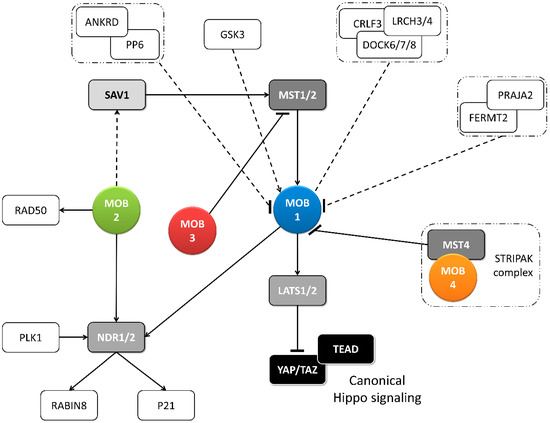
Figure 2. Metazoan MOB proteins present extensive regulation between isotypes. The canonical Hippo pathway is activated by upstream signals resulting in MST1/2 phosphorylation (which may be mediated by SAV1) and MOB1-LATS1/2 activation, causing YAP/TAZ phosphorylation and cytoplasm retention. The lack of YAP/TAZ transcriptional signaling results in a tumor suppressing effect. However, MOB proteins present several activities beyond canonical Hippo signaling. These include non-canonical Hippo signaling through different interactions with GCKII STE20 or NDR kinases, direct MOB stimulation by upstream signals and direct stimulation by MOBs of non-Hippo proteins. This intricate network results in direct and indirect MOB to MOB regulation. PLK1 regulates mitotic spindle orientation through NDR1 phosphorylation which results in NDR1 binding shifting from MOB1 to MOB2, favoring canonical Hippo activation [
48]. NDR1/2 also regulate P21 and RABIN8 [
49,
50]. MOB3 is a MOB1 antagonist by inhibiting MST1 [
36]. MOB4/Phocein-MST4, part of the STRIPAK complex, also antagonizes MOB1, by disrupting MOB1-MST1/1 binding [
34]. MOB1 interacts in a HsMOB1-PPP6R1/2/3-ANKRD28 complex which appears to inhibit MOB1 mediated Hippo activation and in a DOCK6/7/8-CRLF3-LRCH3/4 complex, in a phosphorylation dependent manner [
51,
52]. Both PP6 phosphatase and DOCK6-8 promote actin cytoskeleton polarization signaling via RAC1. The FERMT2-PRAJA2 complex inhibits Hippo signaling by promoting MOB1 ubiquitin-proteasome degradation [
40]. MOB1 is stimulated by GSK3β, a signaling hub involved in Wnt, mTOR, and Notch signaling [
53]. MOB2 interacts with RAD50 stimulating the DNA damage response [
54]. MOB2-SAV1 interaction was detected in
G. gallus [
42]. Notably, PP6, DOCK6, FERMT2 and GSK3 are proteins involved in cell-cell junctions. Colored boxes represent Hippo pathway members while other proteins are represented by non-colored boxes. Protein complexes are identified by an interrupted box with dashes and dots lines. Arrows represent activation while dashes represent inhibition. Lack of arrow or dash indicates an uncertain effect. Full lines represent well-established interactions. Interrupted lines represent less documented interactions.
Table 1. MOB functions in multicellular organisms.
TH—tissue homeostasis, M—morphogenesis, D—differentiation, CC—cell cycle progression, Dm—Drosophila melanogaster, Hs—Homo sapiens, Mm—Mus musculus, Cf—Canis familiaris, Gg—Gallus gallus, At—Arabidopsis thaliana, Ms—Medicago sativa, Nc—Neurospora crassa, An—Aspergillus nidulans, Sm—Sordaria macrospora, Ch—Colletotrichum higginsianum.
2.1.2. Morphogenesis: A MOB Function in Various Species and Cell Types
MOB proteins are also important cell polarity and tissue morphogenesis regulators (
Table 1).
D. melanogaster DmMOB2 interacts with DmNDR (Trc) and is necessary for wing hair, photoreceptor and neuromuscular junction morphogenesis [
66,
67,
68]. DmMOB4/Phocein is also necessary for synapse morphogenesis and microtubule organization [
69]. In
H. sapiens, HsMOB2 loss of function mutations are associated with defective cortical development [
59]. Interestingly, a similar phenotype is produced by mutations in cell adhesion protocadherins FAT4-DCHS1 which is compensated by Yap knockdown [
73].
M. musculus MmMOB1 controls lung morphogenesis through YAP/TAZ regulation and neuritogenesis independent of YAP [
53,
62]. MmMOB1 mediated neuritogenesis is stimulated by GSK3β (glycogen synthase kinase3β) [
53]. Notably, GSK3 functions as a signaling hub, integrated into Wnt, mTOR (mammalian target of rapamycin, and Notch pathways [
74]. MmMOB2 is necessary for neuritogenesis and cortical development, including ciliogenesis [
59,
64]. MmMOB2 RNAi and overexpression studies showed this protein’s role in neuritogenesis is synergistic with MmNDR2 and affects the actin cytoskeleton [
64]. Contrary to
H. sapiens,
M. musculus MmDCHS1 mutation phenotype could not be compensated by YAP knockdown [
59]. MmMOB4/Phocein regulates dendritic arborization in neuronal development through STRIPAK complex signaling [
25]. In
G. gallus, GgSAV1-GgMOB2 interaction also regulates follicle development [
42].
A. thaliana, AtMOB1 is necessary for plant structural development of stem and root [
43,
44,
46]. Also, in fungi, MOB proteins participate in morphogenesis. In
N. crassa NcMOB1 is necessary for septum and aerial mycelium formation and conidiation with Nc
Mob1 gene deletion resulting in increased hyphae branching despite decreased mycelium growth. NcMOB2 controls hyphae polar tip extension through regulation of the NDR kinase NcCOT1 [
3]. NcMOB4/Phocein is essential for vegetative cell fusion and consequent fruiting body formation in a way unrelated to NDR signaling. NcMOB1 and NcMOB4/Phocein both participate in fruiting body morphogenesis but NcMOB1 activity occurs by interaction with NcLATS (DBF2) while NcMOB4/Phocein integrates the STRIPAK complex [
3,
24]. Moreover, NcMOB4/Phocein and SmMOB4/Phocein of the Ascomycete
Sordaria macrospora also control vegetative cell fusion [
3,
23]. These data show that the involvement of MOB proteins in morphogenesis is not exclusive of a specific MOB isotype, but that different MOB isotypes participate in this process (
Table 1).
2.1.3. Cell Cycle Progression: MOBs as Regulators of Mitosis, Cytokinesis, and Centrosome Biology
Several core components of the Hippo pathway have been implicated in the regulation of eukaryotic cell cycle progression, including MOB proteins (
Figure 2 and
Figure 3) [
17]. DmMOB1 null mutants present aberrant chromosome segregation during embryogenesis [
15] while DmMOB4/Phocein depleted cells fail to properly assemble the spindle pole [
70]. DmMOB4/Phocein also integrates the STRIPAK complex and is necessary for PP2A regulated axonal transport of autophagosomes [
71]. DmMOB1 localizes to the cytoplasm and nucleus, but also to the centrosome [
15]. HsMOB1 is required for mitotic exit and its depletion results in prolonged telophase [
13]. The same is true for its binding partner HsLATS1/2. HsMOB1 is necessary for correct positioning of the mitotic regulator chromosomal passenger complex to the spindle midzone during anaphase [
56]. Correct mitotic spindle orientation is regulated through NDR1 phosphorylation by PLK1 which results in NDR1 binding shifting from HsMOB1 to HsMOB2 [
48]. HsMOB1/HsMOB2 competitive binding to NDR1 was also observed in centrosome duplication [
14]. HsMOB2 binding to NDR seems to inhibit its activation which occurs through phosphorylation [
60]. HsMOB1 has also been implicated in centrosome disjunction by interfering with NEK2 centrosome localization [
57]. HsMOB1 also regulates correct cell abscission and cytokinesis [
55]. HsMOB1 knockdown cells show increased motility immediately after telophase/cytokinesis and persist connected by long intercellular bridges [
55]. Also, MOB1 depletion results in centriole separation which supports the idea that MOB1 is required for centriole rejoining at the end of mitosis. Interestingly, components of the Hippo pathway, HsSAV1 and MST2 cooperate with the NEK2 kinase to regulate centrosome disjunction [
57]. Additionally, HsMOB1 interacts with serine/threonine phosphatases PP6 and an ankyrin repeat–containing protein ANKRD, forming a HsMOB1-PPP6R1/2/3-ANKRD28 complex. Also, interactions with leucine-rich repeats and calponin homology domain–containing proteins LRCH, cytokine receptor–like factor 3 and dedicator of cytokinesis proteins DOCK forming a HsDOCK6/7/8-CRLF3-LRCH3/4 complex were described [
51,
52]. Time point analysis suggests the PP6 complex may inhibit HsMOB1 mediated Hippo activation. HsMOB1 is mostly localized in the cytoplasm and to a lesser extent in the cytoplasmic membrane, but it also localizes to various cell cycle associated structures, namely centrosome, kinetochores in early mitosis and spindle midzone in late mitosis [
13,
14,
56,
61]. HsMOB2 induces G1/S cell cycle arrest in response to DNA damage, independently of NDR kinases [
54]. Coincidently, HsMOB2 localizes to the nucleus [
14,
61]. HsMOB3, a cytoplasmic protein, prevents high cell density growth inhibition by downregulating HsMST1 mediated apoptotic signaling in glioblastoma cells [
14,
36]. Similarly to human, in mouse MmMOB1 regulates centrosome duplication in keratinocytes, through the regulation of MmLATS and MmYAP [
37]. MmMOB1 also interacts with MmDOCK8 in thymocytes, stimulating MmRAC1 actin cytoskeleton polarization signaling [
63]. MmMOB1-MmMST1/2 mediate MmRhoA GTP charging and MmRAC1 signaling stimulation.
C. familiaris CfMOB1 is also necessary for correct mitosis in photoreceptor cells [
41]. In plants
A. thaliana and
M. sativa, AtMOB1 and MsMOB1 participate in apoptotic signaling during meiotic microsporogenesis and macrosporogenesis and also regulate cytokinesis [
43,
46,
47]. Supporting its role in cytokinesis MsMOB1 is cytoplasmic, but localizes to the cell plate during septum formation and at spindle microtubule structures related to cytokinesis [
47]. AtMOB1 is present at the cytoplasm, cytoplasmic membrane, and nucleus [
43,
44].
A. thaliana Hippo/MST1/2 homolog SIK1 complements
S. cerevisiae Ste20 deletion in mitotic exit and SIK1 was shown to bind to AtMOB1 [
45]. In conclusion, MOB proteins play critical roles in accurate cell division and cytokinesis and, besides other cellular localizations, tend to localize at the centrosome when this structure is present, a localization that is shared by other Hippo components (
Table 1).
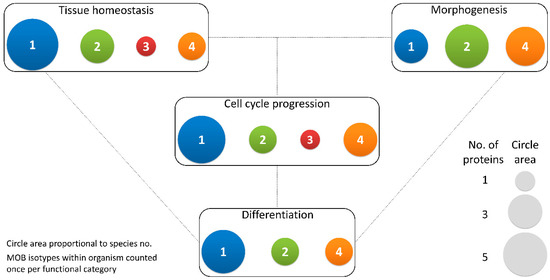
Figure 3. Different MOB isotypes present similar functions. MOB functions were divided into 4 categories: tissue homeostasis, morphogenesis, differentiation and cell cycle progression (includes mitosis, meiosis, cytokinesis, and centrosome biology). Circle areas are proportional to the number of proteins counted in the literature related to these functions, as is illustrated in the protein count examples at the bottom right. MOB protein isotypes for each organism were counted only once per function category (variants within protein isotypes were not considered). It is obvious that there is no one MOB isotype responsible for a particular function. Various MOB isotypes present similar functions, be it in distinct species or in distinct tissues in the same species. In fungi, NcMOB1, ChMOB2, and SmMOB4/Phocein, all participate in conidiation, ascosporogenesis, and meiosis [
3,
4,
23]. In
M. musculus, MmMOB1, MmMOB2, and MmMOB4/Phocein are necessary for neuronal development while MmMOB1 also promotes lung morphogenesis [
25,
53,
62,
64]. The data also evidence a higher abundance of studies on MOB1 proteins, which are the more represented in each category, except morphogenesis. Colors representing MOB isotypes: 1—blue, 2—green, 3—red, 4—orange.
2.1.4. Differentiation and Stem Cell Maintenance: MOB in a Crossroad between Morphogenesis, Tissue Homeostasis, and Cell Cycle Regulation
In
Drosophila DmMOB1 is essential for embryonic development [
15]. This becomes evident after both zygotic and maternal DmMOB1 depletion that results in non-viable embryos indicating a probable role for DmMOB1 in stem cell differentiation. In mouse, MmMOB1 is necessary for embryonic stem cell differentiation into the three germ layers, an activity that is dependent on YAP regulation [
65]. MmMOB1 is also involved in bronchioalveolar cell differentiation and alveolar stem cell maintenance through YAP/TAZ regulation [
62]. Surprisingly, this last study did not find evidence that MmMST or MmLATS kinases were also involved in YAP/TAZ regulation. The plant AtMOB1 is necessary for sexual development, namely during sporogenesis and gametogenesis [
43,
44]. AtMOB1 depleted cells present reduced meristem length and cell number, suggesting a role not only in cell differentiation but also in stem cell maintenance, as documented in mouse [
43,
44,
46]. AtMOB1 depleted cells also present a substantially reduced seed output [
43]. The protein localizes to meiocytes, supporting its participation in meiosis. Interestingly, in
M. sativa MsMOB1 localizes to the meristem in a cell cycle dependent manner [
47]. Fungi MOB proteins are also involved in the differentiation of cells for sexual development and the meiotic process. The NcMOB1,
Colletotrichum higginsianum ChMOB2 and SmMOB4/Phocein are involved in conidiation, ascosporogenesis, and meiosis [
3,
4,
23]. NcMOB2 exhibited similar properties as NcMOB1 in conidiation, but not in ascosporogenesis and meiosis [
3]. ChMOB2 interacts with ChNDR, known as Cbk1 [
4].
A. nidulans AnMOB4/Phocein is also necessary for ascospore production through meiosis [
22].
2.2. Regulation of MOB Proteins
2.2.1. Post-Translation Modifications of MOBs
MOB1 can be regulated by different phosphorylation events coordinated by diverse kinases. In 2008, it was described for the first time that the MST1/2 kinases catalyzed the phosphorylation of HsMOB1 Thr12 and Thr35 amino acid residues and that these post-translation modifications (PTMs) slowed down the cell cycle progression [
27]. Later, a knowledgebase dedicated to mammalian PTMs reported the existence of additional phosphorylation modifications in HsMOB1 amino acid residues, namely in Tyr26, Ser23, and Ser38 [
75]. Recently, it was described that focal adhesion kinase (FAK) regulates YAP by phosphorylating on HsMOB1 Tyr26 residue. This modification results in the dissociation of the functional HsMOB1/LATS complex, thus preventing Hippo-dependent inhibition of YAP [
76]. It is still unclear whether this regulation could also affect the HsMOB1/NDR complex [
18]. On the other hand, the HsMOB1 Ser23 and Ser38 phosphorylation function remains unresolved. However, the HsMOB1 Ser23 represents a possible target site for ATM serine/threonine kinase [
77], suggesting a possible link between HsMOB1 and the DNA damage response (DDR). In fact, HsMOB1 and HsMOB2 were detected in RNAi screens to identify modulators of genome integrity [
78,
79]. HsMOB2 was also identified as a suppressor of homologous recombination in a genome-wide RNAi-based screen and was shown to interact with RAD50, resulting in recruitment of a RAD50 DNA damage sensor complex to damaged chromatin [
54,
80]. Until now, there is no information concerning HsMOB2 phosphorylation. However, HsMOB2 contains several putative ATM phosphorylation sites, which may be related to the DDR function [
77].
Additionally, MmMOB1 Ser146 phosphorylation by the GSK3 kinase (glycogen synthase kinase) was described in the context of neurite outgrowth downstream of the PTEN-GSK3β axis, controlling neurite outgrowth after spinal cord injury [
53].
Similar to HsMOB1, HsMOB3A, HsMOB3B, and HsMOB3C are modified by several phosphorylation events in distinct amino acid residues: Thr15, Thr26, and Ser38 from HsMOB3A; Thr25 and Thr77 from HsMOB3B; Thr14, Thr25, and Ser37 from HsMOB3C [
75]. Although the role of these modifications is unclear, the sequence motifs surrounding Thr15 and Ser38 of HsMOB3A are quite similar to Thr12 and Thr35 of HsMOB1 [
18], raising the hypothesis that HsMOB3A is phosphorylated on these residues by MST1/2 similarly to what has been described for HsMOB1 [
27]. The Ser37 residue of HsMOB3C may represent an ATM targeting site [
77].
The phosphorylation of HsMOB4/Phocein, without any associated regulatory role, has also been observed at Ser147 and Tyr141 residues [
75]. Comparable to the phosphorylation of Ser23 of HsMOB1, of Ser37 of HsMOB3C and putative phosphorylation sites of HsMOB2, the Ser147 phosphorylation of HsMOB4/Phocein is probably performed by the DDR-linked ATM kinase [
77], and will be required for the DNA damage signaling.
No other post-translation modifications were identified in MOB proteins, except that HsMOB2 is ubiquitylated, with ubiquitin linked through at Lys23, Lys32, and Lys131 residues [
81,
82]. The function of these unique PTMs in HsMOB2 are unknown since the fate of a ubiquitylated protein is largely determined by the type of ubiquitin modification with which it is decorated (for review Deol, 2019 [
83]). In human cells, the focal adhesion molecule FERMT2 drives Hippo signaling inhibition by interacting with HsMOB1 and the E3 ligase praja2 and promoting HsMOB1 ubiquitin-proteasome degradation [
53].
2.2.2. Transcriptional and Post-Transcriptional Regulation of MOBs
Data on transcriptional regulation of
Mob genes is scarce, but alteration of DNA methylation patterns has been proposed as a possible mechanism to affect regulation of the levels of MOB proteins and is often associated to cancer. Studies of DNA methylation patterns revealed that lysine demethylase 2B (KDM2B) directly bound to the promoter region of Hs
Mob1 gene, inhibited and promoted pancreatic ductal adenocarcinoma progression [
84]. Furthermore, in a study concerning methylation in human breast cancer in response to resveratrol, HsMOB1 promotor presented methylation changes in triple-negative breast cancer cells [
85]. HsMOB3B transcripts were not downregulated in public data sets, it was also observed that the HsMOB3B promoter region is hypermethylated in a significant number of prostate cancer samples [
86].
In the last years, non-coding RNAs (ncRNAs) have emerged as critical molecules for the post-transcriptional regulation of gene expression. For example, miRNAs have been considered key regulators of target mRNAs by driving its degradation or translational repression. In the case of the Hippo pathway, multiple miRNAs have been described to target members of this via in cancer [
87,
88]. In this context, only three miRNAs were described to target Hs
Mob1 genes: (i) miR-664a-3p, promotes cell proliferation and invasion in gastric cancer [
89]; (ii) miR-135b, promotes migration and invasiveness in lung cancer [
90]; and (iii) miR-181c, promotes tumor progression pancreatic cancer [
91]. Beyond cancer, exosomal miRNAs are important for intercellular communications and functional regulation in recipient cells, being important for several other diseases. Exosomes from human umbilical cord mesenchymal stem cells (HUCMSCs) are effective in inhibiting bone marrow mesenchymal stem cell (BMSC) apoptosis and preventing rat disuse osteoporosis (DOP) by the miR1263/RnMOB1/Hippo signaling pathway [
92].
These few examples on the transcriptional and post-transcriptional regulation of MOB1/Hippo signaling strengthen the need to further understand the molecular mechanisms that regulate/deregulate this pathway. Certainly, it will bring new insights into how this pathway is regulated allowing to define new diagnostic strategies and open new avenues for therapies in, e.g., cancer and developmental diseases.
3. MOB Proteins in Unicellular Organisms: The Roots of Multicellularity?
MOB proteins have been studied in metazoans for more than one decade now, which consolidated their role as signal adaptors that can interact with different kinase families playing critical roles in the Hippo signaling pathway and Hippo-like signaling pathway [
5]. Most studies were carried out in multicellular organisms, strongly suggesting that the Hippo pathway, in crosstalk with other signaling pathways, presents a conserved role throughout the bilaterian animals, thus being a probable hallmark of multicellularity. To trace the evolutionary roots of the Hippo signaling pathway, researchers focused their attention on the presence of genomic sequences coding for the main effector of the pathway, the Yorkie (Yki)/YAP. Genome wide analysis and comparative genome approaches associated with heterologous gene expression studies clearly showed that orthologs of several components of the Hippo pathway, including Yki/YAP, were present in cnidarians (e.g., sea-anemone
Nematostella vectensis), placozoans (e.g.,
Trichoplax adhaerens), and sponges, that are non-bilaterian animals [
93,
94]. Similar studies identified Yki/YAP homologs in two holozoan protists that are assumed to be the closest unicellular relatives of metazoans, namely the filastereans
Capsaspora owczarzaki and the choanoflagellate
Monosiga brevicollis [
95]. Noteworthy, the functional analysis of
C. owczarzaki Hippo components showed that they were able to regulate tissue growth in
Drosophila by activating Yki/YAP [
95]. Based on these data, the authors proposed that the Hippo pathway originated in unicellular organisms within the holozoa, before the divergence of filastereans, choanoflagellates, and metazoans [
95]. Later, Ikmi et al. (2014) [
96], also using heterologous gene expression assays, showed that Yki/YAP proteins from
M. brevicollis,
A. queenslandica,
T. adhaerens,
N. vectensis, and their non-phosphorylatable forms (YAP activity is regulated through phosphorylation by Warts/Lats1,2 kinases) were able to regulate growth in
Drosophila, and to be regulated by phosphorylation, similarly to what is observed for
Drosophila Yki and human YAP.
Currently, most of the core components of the Hippo pathway (e.g., Mats/MOB1, Hippo/MST1/2, and Warts/LATS1/2) have been identified in the genome of animal lineages that diverged before the bilaterians and seem to have similar functions. This suggest that Mats, Hippo, and Warts are evolutionarily more conserved than their upstream regulators (i.e., Fat/Dachsous/Crumbs) and downstream partners in the via (Yki/YAP/TAZ and Scalopped (Sd)/TEAD) [
94]. However, there are still some discrepancies between different studies, for example Yki/YAP was identified in
C. owczarzaki, but bona fide Yki/YAP orthologue is apparently absent from ctenophore genomes [
97]. Nevertheless, atypical sequences may be present in the genome of these organisms. Only TAZ proteins, that are YAP paralogs, were not found in non-vertebrates and seem to have appeared late in evolution. Recently, it was suggested that YAP and TAZ seem to share the same evolutionary origin since TAZ was originated from Yki/YAP during the whole genome duplication in fish, which may explain their redundant roles in the mammalian Hippo pathway [
98]. Although Yki/YAP exhibit a high degree of coevolution with Mats/MOB1 [
93], this last protein is conserved throughout the eukaryotic lineage, whereas Salvador/SAV1, another adaptor of the core pathway, is only present in choanoflagellates and metazoa [
95]. By analyzing the evolutionary history of the Hippo pathway in a variety of unicellular organisms like the ciliate
Tetrahymena thermophila, the centric diatom
Thalassiosira pseudonana, the algae
Chlamydomonas reinhardtii, the flagellated protozoan parasite
Trichomonas vaginalis, and the excavate
Naegleria gruberi, Chen et al. (2020) [
98] concluded that Mats/MOB1 was the earliest member of the Hippo pathway to be identified in these unicellular species. Accordingly, to this study, the Hippo/MDT1/2 kinase was the next member of this via to emerge and was identified in the Amoebozoa (e.g.,
Acanthamoeba castellanii), Dictyostelia (e.g.,
Dictyostelium discoideum), and Apusozoa (e.g.,
Thecamonas trahens). The Warts/LATS1/2 kinase was firstly identified in the cellular slime mold
Fonticula alba and in
S. cerevisiae. The Hippo/Warts/Mats core kinase complex was revealed in the chytrid fungus
Spizellomyces punctatus, whereas the Yki/Sd or YAP/TEAD transcriptional complex was found in the unicellular choanozoan
Corallochytrium limacisporum, an organism belonging to the supergroup Opisthokonta and assumed to be important to understand the evolutionary origin of animals and fungi. Finally, a complete Hippo pathway was attained in
C. owczarzaki [
98] which agrees with the data of Sebé-Pedrós et al. (2012) [
95]. The ancestor components of the Hippo pathway, (i.e., Mats/MOB1, Hippo/MST1/2, and Warts/LATS1/2) from
T. thermophila,
A. castellanii, and
F. alba not only share key domains and regulatory amino acid residues with
C. owczarzaki,
Drosophila, and human orthologs but also present conserved functions in human cells as revealed by heterologous gene expression assays [
98].
The presence of a complete Hippo pathway in a unicellular organism is now undoubtedly accepted but its function is still uncertain. In metazoans, Hippo signaling plays critical roles during development and is in crosstalk with other signaling cascades, for example Notch, Hedgehog, and Wnt signaling, all absent in unicellular organisms. Sebé-Pedrós et al. (2012) [
95] proposed that the Hippo pathway in unicellular organisms may be involved in the coordination of cell proliferation in response to cell density or cell polarity. This assumption was based on the fact that the metazoan apical proteins Merlin/NF2 (recognized as a critical mediator of contact inhibition of proliferation and regulator of Hippo pathway), Kibra (functions as a scaffold protein in various cell processes, such as cell polarity, cell migration, and membrane trafficking [
99]), aPKC kinase (plays a critical role in the regulation of apical-basal polarity in epithelial cells and asymmetrically dividing cells [
100]) and Lethal-2-giant larvae (Lgl, makes part of the Scribble cell polarity module involved in cell polarity, control of tissue growth, differentiation and directed cell migration [
101]) are encoded in the
C. owczarzaki genome [
95]. Moreover, recent studies in ciliates showed that some Hippo proteins link accurate cell division and cytokinesis to morphology [
6]. In unicellular organisms, the maintenance of morphology likely relies more on self-organization than in extrinsic/intrinsic cues as it occurs in metazoa, with the Hippo proteins having a crucial role in the perpetuation of cell pattern and organization. Thus, Hippo signaling research in unicellular organisms may reveal the ancestral regulatory routes of cell division and cell number control in metazoans
Probably, the establishment of a tight regulation control may have contributed to the establishment of multicellularity. Studies in unicellular organisms will allow for the definition of core and pivotal conserved modules of these signaling pathways. Consequently, in the next sections, we will focus our attention on the role of MOB proteins in two groups of unicellular organisms where the functional role of this protein has been largely studied, namely the yeast and members of the alveolate clade.