Angiogenesis, the generation of new blood vessels, is one of the hallmarks of cancer. The growing tumor requires nutrients and oxygen. Recent evidence has shown that tumors release signals to attract new nerve fibers and stimulate the growth of new nerve fibers. Neurogenesis, neural extension, and axonogenesis assist in the migration of cancer cells. Cancer cells can use both blood vessels and nerve fibers as routes for cells to move along. In this way, neurogenesis and angiogenesis both contribute to cancer metastasis. As a result, tumor-induced neurogenesis joins angiogenesis and immunosuppression as aberrant processes that are exacerbated within the tumor microenvironment. The relationship between these processes contributes to cancer development and progression. The interplay between these systems is brought about by cytokines, neurotransmitters, and neuromodulators, which activate signaling pathways that are common to angiogenesis and the nervous tissue. These include the AKT signaling pathways, the MAPK pathway, and the Ras signaling pathway. These processes also both require the remodeling of tissues. The interplay of these processes in cancer provides the opportunity to develop novel therapies that can be used to target these processes.
- cells cells
- axonogenesis
- metastasis
- cancer
1. Introduction
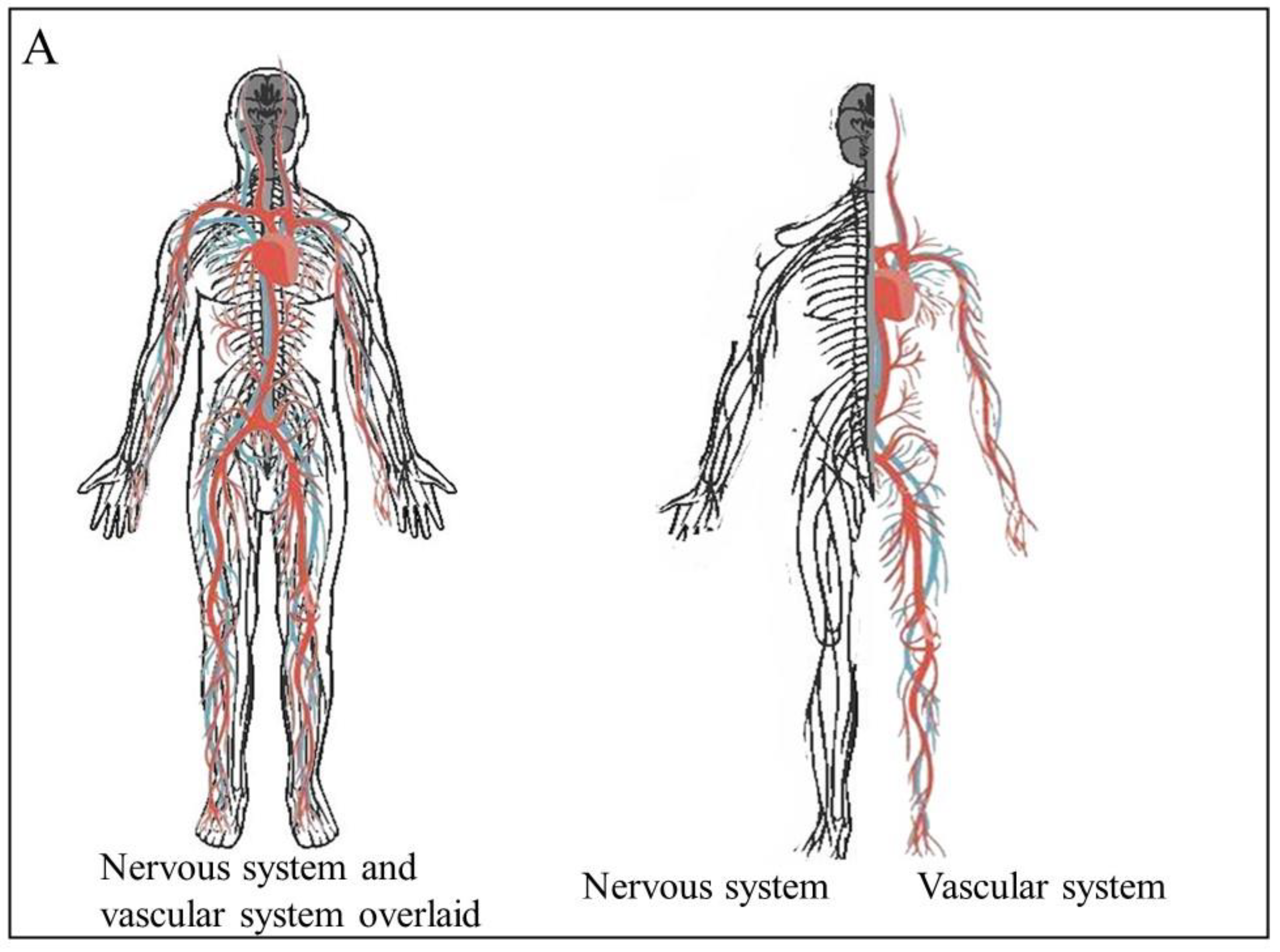
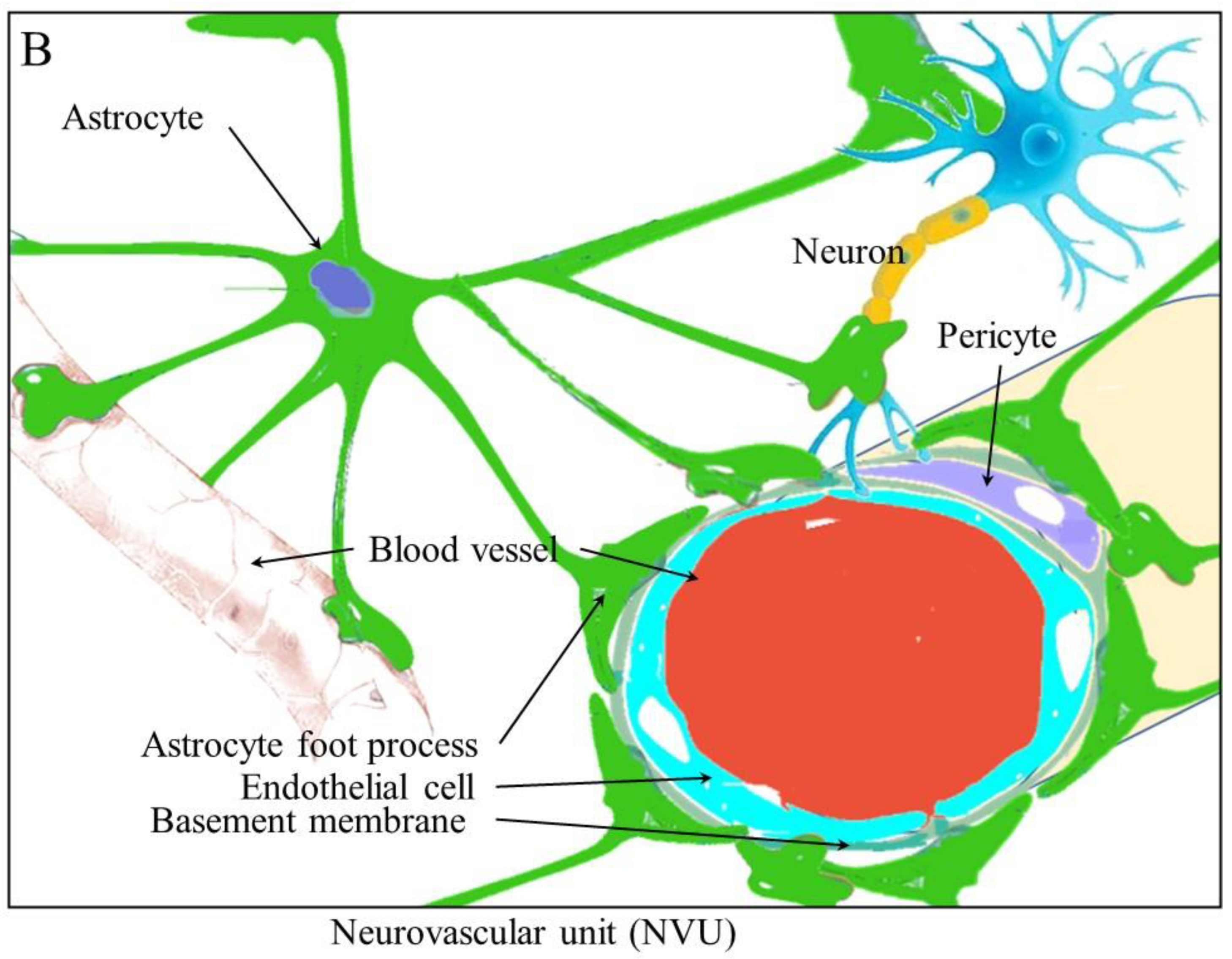
2. Angiogenesis and Neurogenesis
3. The Remodeling of Tissues and the Proliferation and Migration of Cells during Angiogenesis and Neurogenesis
3.1. Migration and Remodeling
3.2. Stromal-Cell-Derived Factor-1 and CXC Chemokine Receptor 4
3.3. Monocyte Chemoattractant Protein-1
3.4. Matrix Metalloproteinases
4. Therapeutic Targeting of the Interplay between Angiogenesis and Neurogenesis
This entry is adapted from the peer-reviewed paper 10.3390/cancers15061805
References
- Cao, Y.; Arbiser, J.; D’Amato, R.J.; D’Amore, P.A.; Ingber, D.E.; Kerbel, R.; Klagsbrun, M.; Lim, S.; Moses, M.A.; Zetter, B.; et al. Forty-year journey of angiogenesis translational research. Sci. Transl. Med. 2011, 3, 114rv113.
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front. Physiol. 2012, 3, 97.
- Deng, J.; You, Q.; Gao, Y.; Yu, Q.; Zhao, P.; Zheng, Y.; Fang, W.; Xu, N.; Teng, L. Prognostic value of perineural invasion in gastric cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88907.
- Schmitd, L.B.; Scanlon, C.S.; D’Silva, N.J. Perineural Invasion in Head and Neck Cancer. J. Dent. Res. 2018, 97, 742–750.
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361.
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603.
- Bapat, A.A.; Hostetter, G.; Von Hoff, D.D.; Han, H. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer 2011, 11, 695–707.
- Carmeliet, P.; Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 2005, 436, 193–200.
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41.
- Font, M.A.; Arboix, A.; Krupinski, J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr. Cardiol. Rev. 2010, 6, 238–244.
- Eisenberg, E.; Suzan, E. Drug combinations in the treatment of neuropathic pain. Curr. Pain Headache Rep. 2014, 18, 463.
- Slavik, E.; Ivanović, S.; Grujicić, D. Cancer pain (classification and pain syndromes). Acta Chir. Iugosl. 2004, 51, 9–14.
- Schmidt, B.L. The neurobiology of cancer pain. Neuroscientist 2014, 20, 546–562.
- Kopp, H.G.; Ramos, C.A.; Rafii, S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr. Opin. Hematol. 2006, 13, 175–181.
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907.
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693.
- Luo, Z.; Shang, X.; Zhang, H.; Wang, G.; Massey, P.A.; Barton, S.R.; Kevil, C.G.; Dong, Y. Notch Signaling in Osteogenesis, Osteoclastogenesis, and Angiogenesis. Am. J. Pathol. 2019, 189, 1495–1500.
- Wong, P.P.; Bodrug, N.; Hodivala-Dilke, K.M. Exploring Novel Methods for Modulating Tumor Blood Vessels in Cancer Treatment. Curr. Biol. 2016, 26, R1161–R1166.
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307.
- Lee, H.W.; Xu, Y.; He, L.; Choi, W.; Gonzalez, D.; Jin, S.W.; Simons, M. Role of Venous Endothelial Cells in Developmental and Pathologic Angiogenesis. Circulation 2021, 144, 1308–1322.
- Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337.
- Chen, H.; Liu, D.; Yang, Z.; Sun, L.; Deng, Q.; Yang, S.; Qian, L.; Guo, L.; Yu, M.; Hu, M.; et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocr. -Relat. Cancer 2014, 21, 783–795.
- Huang, T.; Tworoger, S.S.; Hecht, J.L.; Rice, M.S.; Sood, A.K.; Kubzansky, L.D.; Poole, E.M. Association of Ovarian Tumor β2-Adrenergic Receptor Status with Ovarian Cancer Risk Factors and Survival. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1587–1594.
- Gong, C.; Hu, B.; Chen, H.; Zhu, J.; Nie, J.; Hua, L.; Chen, L.; Fang, Y.; Hang, C.; Lu, Y. β2-adrenergic receptor drives the metastasis and invasion of pancreatic ductal adenocarcinoma through activating Cdc42 signaling pathway. J. Mol. Histol. 2022, 53, 645–655.
- Ray, R.; Al Khashali, H.; Haddad, B.; Wareham, J.; Coleman, K.L.; Alomari, D.; Ranzenberger, R.; Guthrie, J.; Heyl, D.; Evans, H.G. Regulation of Cisplatin Resistance in Lung Cancer Cells by Nicotine, BDNF, and a β-Adrenergic Receptor Blocker. Int. J. Mol. Sci. 2022, 23, 12829.
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.-H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-Coupled Astrocytic Differentiation and Age-Related Depletion of Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell 2011, 8, 566–579.
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014, 344, 630–634.
- Ponti, G.; Obernier, K.; Alvarez-Buylla, A. Lineage progression from stem cells to new neurons in the adult brain ventricular-subventricular zone. Cell Cycle 2013, 12, 1649–1650.
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.-L.; Song, H. In Vivo Clonal Analysis Reveals Self-Renewing and Multipotent Adult Neural Stem Cell Characteristics. Cell 2011, 145, 1142–1155.
- Livneh, Y.; Adam, Y.; Mizrahi, A. Odor Processing by Adult-Born Neurons. Neuron 2014, 81, 1097–1110.
- Fuentealba, L.C.; Obernier, K.; Alvarez-Buylla, A. Adult Neural Stem Cells Bridge Their Niche. Cell Stem Cell 2012, 10, 698–708.
- Dranovsky, A.; Picchini, A.M.; Moadel, T.; Sisti, A.C.; Yamada, A.; Kimura, S.; Leonardo, E.D.; Hen, R. Experience Dictates Stem Cell Fate in the Adult Hippocampus. Neuron 2011, 70, 908–923.
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 2008, 13, 717–728.
- Thaker, P.H.; Sood, A.K. Neuroendocrine influences on cancer biology. Semin. Cancer Biol. 2008, 18, 164–170.
- Armaiz-Pena, G.N.; Lutgendorf, S.K.; Cole, S.W.; Sood, A.K. Neuroendocrine modulation of cancer progression. Brain Behav. Immun. 2009, 23, 10–15.
- Marchesi, F.; Piemonti, L.; Mantovani, A.; Allavena, P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010, 21, 77–82.
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440.
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 414.
- Vázquez-Prado, J.; Bracho-Valdés, I.; Cervantes-Villagrana, R.D.; Reyes-Cruz, G. Gβγ Pathways in Cell Polarity and Migration Linked to Oncogenic GPCR Signaling: Potential Relevance in Tumor Microenvironment. Mol. Pharmacol. 2016, 90, 573–586.
- Venneri, M.A.; De Palma, M.; Ponzoni, M.; Pucci, F.; Scielzo, C.; Zonari, E.; Mazzieri, R.; Doglioni, C.; Naldini, L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 2007, 109, 5276–5285.
- Li, F.; Zhao, Y.; Wei, L.; Li, S.; Liu, J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol. Ther. 2018, 19, 695–705.
- Saloman, J.L.; Albers, K.M.; Rhim, A.D.; Davis, B.M. Can Stopping Nerves, Stop Cancer? Trends Neurosci. 2016, 39, 880–889.
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669.
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408.
- Da Cunha, B.R.; Domingos, C.; Stefanini, A.C.B.; Henrique, T.; Polachini, G.M.; Castelo-Branco, P.; Tajara, E.H. Cellular Interactions in the Tumor Microenvironment: The Role of Secretome. J. Cancer 2019, 10, 4574–4587.
- Mantyh, P.W.; Koltzenburg, M.; Mendell, L.M.; Tive, L.; Shelton, D.L. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011, 115, 189–204.
- Kusakabe, T.; Sawaji, Y.; Endo, K.; Suzuki, H.; Konishi, T.; Maekawa, A.; Murata, K.; Yamamoto, K. DUSP-1 Induced by PGE(2) and PGE(1) Attenuates IL-1β-Activated MAPK Signaling, Leading to Suppression of NGF Expression in Human Intervertebral Disc Cells. Int. J. Mol. Sci. 2021, 23, 371.
- Jimenez-Andrade, J.M.; Mantyh, P.W. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res. Ther. 2012, 14, R101.
- Zhang, R.L.; Zhang, Z.G.; Lu, M.; Wang, Y.; Yang, J.J.; Chopp, M. Reduction of the cell cycle length by decreasing G1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J. Cereb. Blood Flow Metab. 2006, 26, 857–863.
- Götz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 777–788.
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326.
- Xia, Y.; Wei, Y.; Li, Z.Y.; Cai, X.Y.; Zhang, L.L.; Dong, X.R.; Zhang, S.; Zhang, R.G.; Meng, R.; Zhu, F.; et al. Catecholamines contribute to the neovascularization of lung cancer via tumor-associated macrophages. Brain Behav. Immun. 2019, 81, 111–121.
- Yamashita, T.; Ninomiya, M.; Acosta, P.H.; García-Verdugo, J.M.; Sunabori, T.; Sakaguchi, M.; Adachi, K.; Kojima, T.; Hirota, Y.; Kawase, T.; et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci. 2006, 26, 6627–6636.
- Sawada, A.; Niiyama, Y.; Ataka, K.; Nagaishi, K.; Yamakage, M.; Fujimiya, M. Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain 2014, 155, 1762–1772.
- Kokovay, E.; Goderie, S.; Wang, Y.; Lotz, S.; Lin, G.; Sun, Y.; Roysam, B.; Shen, Q.; Temple, S. Adult SVZ Lineage Cells Home to and Leave the Vascular Niche via Differential Responses to SDF1/CXCR4 Signaling. Cell Stem Cell 2010, 7, 163–173.
- Hattori, F.; Murayama, N.; Noshita, T.; Oikawa, S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J. Neurochem. 2003, 86, 860–868.
- Robin, M.; Andreu-Gallien, J.; Schlageter, M.H.; Bengoufa, D.; Guillemot, I.; Pokorna, K.; Robert, C.; Larghero, J.; Rousselot, P.; Raffoux, E.; et al. Frequent antibody production against RARalpha in both APL mice and patients. Blood 2006, 108, 1972–1974.
- Wang, T.; Choi, E.; Monaco, M.C.; Major, E.O.; Medynets, M.; Nath, A. Direct induction of human neural stem cells from peripheral blood hematopoietic progenitor cells. J. Vis. Exp. 2015, 95, e52298.
- Katakowski, M.; Zhang, Z.G.; Chen, J.; Zhang, R.; Wang, Y.; Jiang, H.; Zhang, L.; Robin, A.; Li, Y.; Chopp, M. Phosphoinositide 3-kinase promotes adult subventricular neuroblast migration after stroke. J. Neurosci. Res. 2003, 74, 494–501.
- Barkho, B.Z.; Munoz, A.E.; Li, X.; Li, L.; Cunningham, L.A.; Zhao, X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells 2008, 26, 3139–3149.
- Guo, Z.; Liu, D.; Su, Z. CIP2A mediates prostate cancer progression via the c-Myc signaling pathway. Tumour Biol. 2015, 36, 4777–4783.
- Hoeppner, L.H.; Sinha, S.; Wang, Y.; Bhattacharya, R.; Dutta, S.; Gong, X.; Bedell, V.M.; Suresh, S.; Chun, C.; Ramchandran, R.; et al. RhoC maintains vascular homeostasis by regulating VEGF-induced signaling in endothelial cells. J. Cell Sci. 2015, 128, 3556–3568.
- Gao, J.; Zhang, C.; Gao, F.; Li, H. The effect and mechanism of dopamine D1 receptors on the proliferation of osteosarcoma cells. Mol. Cell. Biochem. 2017, 430, 31–36.
- Al-Salama, Z.T.; Syed, Y.Y.; Scott, L.J. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs 2019, 79, 665–674.
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115.
