You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Precision oncology can be defined as molecular profiling of tumors to identify targetable alterations.
- precision medicine
- precision oncology
- pharmacogenomics
- type II endometrial cancer (ECa)
- prostate cancer (PCa)
1. Prostate Cancer (PCa)
1.1. Diagnosis and Risk Factors
Prostate cancer is often only diagnosed at a very late stage because the initial stages of the disease have no symptoms [30]. If symptoms do occur, they include dull pain in the lower abdomen; frequent urination; pain during urinating; blood in the urine; pain during ejaculation, loss of weight and appetite and bone pain. The most common screening tests for prostate cancer are the digital rectal exam (DRE) and prostate specific antigen (PSA) blood test [31]. The DRE is a physical examination of the prostate, where the size and shape or thickness of the prostate can give an indication of prostate cancer. While the test is easily performed and cost effective, it may not be able to detect early-stage prostate cancer [32]. PSA is produced by the prostate and is over-produced by prostate cancers. The PSA blood test measures the level of PSA in the blood, with high levels indicating possible prostate cancer. Despite the test being easy to perform and relatively cheap, it does not provide any information of the type of cancer and cannot distinguish between prostate cancer, benign enlargement of the prostate or inflammation of the prostate [32,33].
Once these initial studies have indicated the presence of PCa, further diagnostic tests can be carried out and these include transrectal ultrasound, magnetic resonance imaging (MRI) or prostate biopsy. These techniques require specialised equipment and trained practitioners and may be limited in LMICs [34]. The aggressiveness of the PCa is commonly determined through genomic testing, where the presence of specific genetic mutations can indicate the aggressiveness of the cancer [31]. However, many of the specific genes these tests look for are based on research performed on non-African individuals, and those of African ancestry may have different genetic mutations that can drive the aggressiveness of the cancer [32,33].
Environmental factors such as diet have been implicated in contributing to PCa incidence. Men who emigrate from a country with a low incidence of PCa tend to develop PCa at the same rate as men in their adopted country. This implicates the change in environment between the native low incidence country to that of the high incidence adopted country as a risk factor [35]. One of the most obvious changes in the environment would be the change in diet. The Western diet is associated with higher PCa risk and is high in fat, red meat, alcohol, and dairy products [36]. High meat intake is suspected to play a role in more aggressive PCa while increased fruit and whole grain food is associated with decreased PCa risk [37]. Obesity is suspected to contribute to the progression of PCa as there is a correlation between PCa progression and body mass index [35,36,37,38]. It is thought that this progression of PCa and increased incidence of PCa linked with obesity is due to the hormone changes induced by excess fat deposits [38].
1.2. Genomics, Racial and Socioeconomic Disparities
Therapeutic clinical trials for men with PCa have considerably increased over the past 16 years [39]. However, one of the obstacles impeding significant progress is the lack of adequate representation of other populations such as those of African ancestry in general medical research (Figure 3A). In a recent paper describing the methods of representing genome wide association study (GWAS) data, a summary of the race of the participants in these studies showed the racial disparities in these recent GWAS. This showed an obvious bias towards white or European populations in these studies. This is represented in Figure 3A. In addition to this, the enrolment of participants in the clinical trials for three FDA approved drugs specifically for PCa treatment is shown in (Figure 3B). The data in Figure 3B show the percentage of each racial group enrolled in these clinical trials. For instance, PCa-centered studies in the USA have revealed the unwillingness of African American men to partake in such clinical research given the opportunity. Concerns over transparency and relevance to cultural contexts need to be considered for adequate inclusion [40].
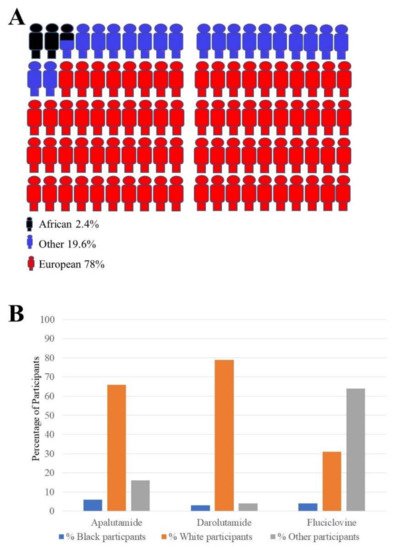
Figure 3. Racial disparitis in genome wide association studies (GWAS) and clinical trials enrolment: (A) Different populations’ contributions to new genomic related discoveries. Compared to other populations such the European group or other population groups, there is little participation of the African population in genomic related studies. These numbers come from participants taking part in a large number of studies (n = 110291); (B) summary of enrolment of black and white men in representative clinical trials for three Food and Drug Administration (FDA)-approved PCa drugs. The number of white participants enrolled for all three PCa FDA approved drugs (2016–2019) appears to be higher than the number of black participants in all three studies. (Apalutamide study n = 1207) (Darolutamide study n = 1509) (Fluciclovine study n = 596).
The United Nations (UN) has developed the Human Development Index (HDI) as a statistic to measure a country’s level of social and economic development. The HDI is made up of various measurements, The mean years of education, life expectancy and gross national income per capita [41]. A study performed by Sharma in 2019 to identify if there is a relationship between HDI and the burden of prostate cancer, in the form of mortality-to-incidence ratio, used pairwise correlation and bivariate regression [30]. This was performed in 87 countries, in the period 1990–2016. It was found that countries with a lower HDI had higher mortality and lower survival rates. However, the mortality rate was shown to decrease over the period of the study. This is probably due to advancements in screening and treatment which have become available even in low income countries [30].
In precision oncology, the best interpretation of genomic results is achieved through multidisciplinary input. This also reduces bias and uncertainty in the clinical data [3]. Many genetic studies have identified PCa being linked to specific loci, Table 1. The majority of these studies were once again initially performed in men of European or Asian descent and many of these described loci have not been identified as being linked to PCa in men of African descent (PCa-African loci) [42,43,44,45,46,47]. Some of the loci associated with PCa in men of European descent showed much lower effects, no effects, or even completely opposite effects in men of African ancestry [48]. However, many of the PCa associated loci have been identified as being shared between men with PCa of both European and African ancestry. These include 8q24, 3p12 [43], KLK2/3 (19q13.33), NUDT10/11 (Xp11.22) [42], 11q13.2, HNF1B/TCF2 (17q12) [49], JAZF1, and MSMB [50]. Table 1 shows the PCa population linked genetic loci.
Table 1. Prostate cancer population risk associated loci.
| Gene Marker | Gene Product Role | Loci | Ref. |
|---|---|---|---|
| European only | |||
| CABP | Calcium-binding protein 1 | 1p36 | [51] |
| HOXB13 rs138213197 | Homeobox protein Hox-B13 | 17q21 | [52] |
| European and African American | |||
| HPC20 | hereditary prostate cancer genetic-susceptibility locus | *20q13 | [53,54] |
| HPC1 | hereditary prostate cancer genetic-susceptibility locus | *1q24-25 | [53,54,55,56] |
| PCAP | Predisposing for Cancer Prostate locus | *1q42-43 | [53,54,56] |
| HPCX | Hereditary Prostate Cancer, X-Linked | *Xq27-28 | [54,57] |
| *8q24 | [43] | ||
| *3p12 | [43] | ||
| KLK2/3 | Kallikrein-2/3 | *19q13.33 | [42] |
| NUDT10/11 | Nucleoside diphosphate-linked moiety X motif 10/11 (Nudix motif 10/11) | *Xp11.22 | [49] |
| 11q13.2 | [49] | ||
| HNF1B/TCF2 | Hepatocyte nuclear factor 1-beta/Transcription gactor 2 | 17q12 | [49] |
| JAZF1 | Juxtaposed with another zinc finger protein 1 | [50] | |
| MSMB | Beta-microseminoprotein | [50] | |
| African American | |||
| DXS986 | DExD/H-Box Helicase 58 | *Xq21 | [58] |
| D17S1852 | Microsatellite marker | *17p11 | [58] |
| rs980481 | A/C/T single-nucleotide variation on chromosome 2 | *2p16 | [59] |
| rs71527 | C/T single nucleotide variation affecting the gene coding for Carbamoyl-phosphate synthase 1 (CPS1) | *2p16 | [59] |
| rs11067228 | A/G single-nucleotide variation on chromosome 12 | *12q24 | [59] |
| D11S908 | DNA segment containing a CA repeat | *11q22 | [58] |
| D2S2259 | DNA segment containing a CA repeat | *2p21 | [58] |
* PCa loci linked to African ancestry.
Previous studies involving varied populations, but still mainly European men, have identified genes that are associated with PCa include those involved in DNA damage and repair, carcinogen metabolism, inflammation and steroid hormone metabolism [60,61,62,63,64,65]. Specific genes with mutations and expression changes associated with PCa include androgen receptor (AR), telomerase-related genes (TERT, TET) [66], genes involved in carcinogen metabolism such as UDP-glucuronosyltransferase 1–8 (UGT1A8) and cytochrome P45021A2 (CYP21A2), metalloproteinase genes and various non-coding RNAS (ncRNAs), including micro-RNAs (miRNAs) and long non coding RNAs (lncRNAs) [67]. By studying and comparing the mutation profiles of 474 genes in different stage tumors from patients of European, African and Asian ancestry, Mahal et al. noted that men of African descent had higher rates of forkhead box protein A1 (FOXA1) mutations [68]. FOXA1 is a transcription factor that is responsible for tissue-specific gene expression and regulation of gene expression in differentiated tissues. Therefore, mutations that could potentially affect the function of this transcription factor in cancer is not surprising. This study also identified that African men with metastatic PCa had higher rates of mutations in androgen receptor genes as well as genes involved in DNA-repair [68].
A similar study comparing mutation profiles in men of African and European ancestry with PCa was conducted. This study focused on genes involved in immune-oncogenic pathways. A race specific gene expression profile was identified with 38 differentially expressed genes specific to each race group. These genes were involved in immune-oncogenomic pathways such as cytokine signaling, interferon (IFN) signaling (IFNγ and IFNα responses), apoptosis, nuclear factor NF-kB (NF-kB) signaling in the tumor necrosis factor alpha (TNFα) pathway, epithelial–mesenchymal transition (EMT) pathways, and signaling by ILs including IL4 and IL13. This implies that immune related pathway alteration is more prevalent in men of African ancestry with PCa [69].
Testosterone metabolism plays an important role in PCa and the cytochrome P450 enzymes involved in this metabolic pathway show high levels of allelic variations depending on ethnicity and ancestry [70,71]. The androgen receptor, a well- known molecular participant in PCa, was found to have different polymorphisms which occur at different frequencies in men with PCa depending on ethnicity and ancestry. These polymorphisms involve high-frequency repeats that occur in the amino-terminal. Men of African ancestry typically have shorter CAG repeats [72]. Race specific changes in the DNA methylation levels of certain PC were also identified in malignant PCa [73,74]. This leads to gene silencing, and higher rates of methylation were observed on the regions coding for CD44 and glutathione S-transferase P (GSTP1) in men of African ancestry with PCa. The silencing of these genes is associated with increased risk of PCa [74,75].
2. Endometrial Cancer (ECa)
2.1. Diagnosis and Risk Factors
The symptoms of endometrial cancer include post-menopausal bleeding, bleeding between periods, pelvic pain and abnormal discharge. It is advised that women with these symptoms should be screened, but there is no evidence that asymptomatic women should be screened [76]. High risk patients should be screened annually after the age of 35 [76]. Physical examination should initially be performed to eliminate any other causes of the symptoms. This can be followed by the most common and recommended diagnostic tests, transvaginal ultrasonography with endometrial biopsy. Transvaginal ultrasonography is commonly available and cheap and sensitive. It measures endometrial thickness and any measurement of a thickness higher than 5 mm is an indicator of endometrial pathology [77]. Endometrial tissue biopsy is the most reliable diagnostic test; however, it may not always be easy to obtain a sufficient sample. Options to increase tissue sampling include dilatation and curettage (D&C), through the use of a curette. Sampling or diagnosis can be achieved with a specialized sampling tool known as the Pipelle. This is accurate and cost effective [78]. Other diagnostic options include hysteroscopy and, although MRI, positron emission tomography (PET) and computed tomography (CT) scans are also useful diagnostic tools, they are expensive and may not be readily available in low income countries [77].
Even in higher income countries, such as the U.S.A., patient mortality and positive treatment outcome rely on the early diagnosis of endometrial cancer. For instance, a comparison between the mortality rates in rural and urban endometrial cancer patients in Utah showed that rural women had a higher mortality rate as a result of a later diagnosis of the disease. This is most likely due to screening facilities not being readily available in rural areas, requiring patients to take more time and effort, travelling a greater distance [79].
In African American communities, the best predictors of endometrial cancer related death (survival) were early stage diagnosis. This was followed by family income and the financial well-being of the family and body mass index (BMI). However, further analysis revealed that the only stage of the cancer and BMI are accurate predictors of patient survival [80]. BMI is related to socioeconomic status while obesity (high BMI) is associated with increased risk of developing cancer and increased mortality [81]. High BMI is also associated with increased mortality following surgical treatment of endometrial cancer [82].
2.2. Genomics, Racial and Socioeconomic Disparities
Histologically, ECa is classified into either type I, estrogen dependent with a better outcome or type II, estrogen-independent with a poor prognosis. However, recently, histopathological and molecular reports have indicated a rather complex ECa risk stratification approach [83]. Results from The Cancer Genome Atlas (TCGA) research network have established four distinctive molecular subtypes: ultra-mutated, hyper-mutated, copy-number low and copy-number high [23,83]. Each of these four sub-types reflect the underlying molecular alterations and associated clinical phenotypes. The recent molecular classification of ECa presents with better opportunities to understand ECa tumor biology, differentiated risk stratification, improved prognosis and improved estimation of the responses of a patient to therapy [23,83]. The ultimate goal of this new risk stratification is its integration into clinical settings and thereby create a foundation for precision oncology in ECa patient care, particularly type II ECa [23]. Type II ECa survival and disease have been shown to be correlated with household income, with women from households with a higher income presenting with less aggressive forms of the disease, disease at an earlier stage and increased survival [84]. One useful indicator of the socioeconomic status of an individual is their level of education. As such, it was also found that those women with higher levels of education are less likely to only be diagnosed at the later stages of the disease and are more likely to get effective treatment and consequently have higher rates of survival [85]. This socioeconomic effect is amplified in poor black women, who are more than twice as likely to die from type II ECa. Apart from any role played by genetics or family history, this is most likely due to the fact that Black women more often receive treatment at a much later stage of disease [86].
Although recent advances have identified a number of molecular targets, which are currently being explored for effective treatment of type II ECa, there is still a lack of novel biomarkers and therapeutic targets. P53 mutations have been reported in 57.7–92% of type II ECa [87].
Studies have identified multiple genes whose expression changes in type II ECa. Oncogenes whose expression changes include GTPase kras (KRAS), human epidermal growth factor receptor 2 (HER2), epithelial growth factor receptor (EGFR), phosphatidylinositol 3-kinase catalytic subunit (PI3KCA) and fibroblast growth factor receptor 2 (FGFR2). Additionally, the expression of tumor suppressors, such as PTEN, p53, p21 and cyclin-dependent kinase inhibitor 2A (CDKN2A), is also altered in type II ECa. Other genes whose expression is altered in type II ECa include genes involved in apoptosis, genes involved in DNA mismatch repair and genes coding for hormone receptors (BCL 2, hMLH1, hMSH2, hMSH6, PMS1 and PMS2, ER and PR)) [88,89].
The change in the expression of some genes is so marked that they can be used as markers for ECa; these include the proliferation marker Ki-67 and angiogenesis growth factors (VEGF-A) [90]. More ECa include genetic alterations in p53, HER2, p16 and E-cadherin [91]. The use of changes in p53 expression was particularly noticeable in African American ECa patients compared with those of European ancestry [91]. As recently discovered functional parts of the human genome, the non-coding RNAs (ncRNAs) including microRNAs and long-ncRNAs have been reported to play a role in tumor development, progression and drug resistance. Furthermore, the ncRNA signatures in distinct races in PCa and ECa remain to be elucidated [92,93].
3. Non-Coding RNAs in PCa and ECa
3.1. PCa and Type II ECa Associated Micro-RNAs (miRNAs) in African Population
MicroRNAs (miRNAs) act as regulators of gene expression by binding to specific mRNAs and inhibiting or modifying their translation. They are able to target specific mRNAs by binding to complementary regions in the 3’ untranslated region (UTR) of the target mRNAs [94] (Figure 4). Aberrant miRNA expression has been observed in PCa [95,96] and ECa [21]. In both cancers, these miRNAs can lead tumor progression or tumor suppression.
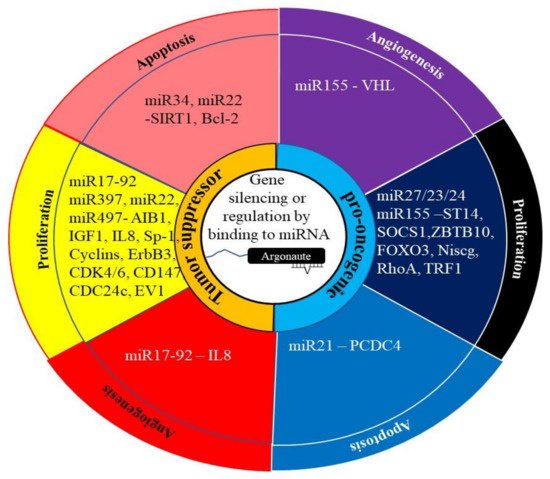
Figure 4. The role of miRNA in cancer. The regulation of gene expression by miRNAs generally involves them binding to their target mRNA and preventing or altering its translation into protein. This can be achieved through the degradation of the mRNA or altering splicing of the mRNA. In cancer, these miRNAs can act as pro-oncogenic by targeting tumor suppressor genes. Alternately, they can act as tumor suppressor miRNAs by targeting the miRNA of oncogenes.
The PCa differences in the miRNA profiles of men of European ancestry and men of African ancestry have been identified that are related to the occurrence, prognosis and progression of PCa [97]. Five miRNAs were identified to have different expression in men of African and European ancestry. These were miR-1b, miR-26a, miR-30c-1, miR-219 and miR-301 [97]. MiRNA26a expression increased in African American non-malignant, malignant, and metastatic prostate cancer cells, compared to European non-malignant, malignant, and metastatic prostate cancer cells. The expression of the miRNA increased in the cell lines of both African and European origin as the malignancy of the cells increased [98,99]. Decreased miR-26a levels lead to cell cycle arrest at G2/M phase followed by caspase 3/7 activation [100]. PCA patients of African ancestry and European ancestry have also exhibited differential expression of let7c and miR-30c [101]. Wang et al. identified miRNAs whose expression is specific to men of African ancestry, and the genes they regulate include miR-133a/MCL1, miR-513c/STAT1, miR-96/FOXO3A, miR-145/ITPR2, and miR-34a/PPP2R2A. The results of the study also suggested that the changes in these miRNAs activate EGFR–PI3K–AKT signaling pathways while knockdown of these miRNAs results in decreased proliferation, aggression and sensitivity to docetaxel-induced cytotoxicity [102].
A study examining the differences in miRNA profiles between healthy women and women with type II ECa, identified 280 miRNAs whose expression was different in the two groups. Women of African ancestry with ECa had increased expression of miR-1269b and decreased expression of miR-1269a, miR-891a and miR-892a compared to women of European ancestry [21]. The miRNA hsa-miR-337-3p was found to be downregulated more often in women of European ancestry with type II ECa [103]. The decrease in the levels of this miRNA is associated with lymph node metastasis in cancers such as gastric cancer [104].
3.2. PCa and Type II ECa Associated Long Non-Coding RNAs (lncRNAs) in the African Population
Long non-coding RNAs (lncRNAs), which are transcripts that are >200 nucleotides long and have no protein-coding potential, have emerged as important targets in tumorigenesis and tumor progression studies. Aberrations in the transcription profiles of various lncRNAs have been shown to be the driving force behind several cancer phenotypes [105]. They do this through their interactions with other components of the cell such as proteins, DNA and other RNA molecules. Increasing evidence shows that lncRNAs have great potential to be diagnostic and prognostic biomarkers because they are expressed in a tissue, cell type and cancer type-specific manner [106] (Figure 5). LncRNAs are detectable in bodily fluids and cancer samples; therefore, they have appreciable value as diagnostic tools and as potential biomarkers [107]. The most widely used test for the detection of prostate cancer, the prostate-specific antigen (PSA) test, can produce false positives or negatives as several other disorders (e.g., benign prostatic hyperplasia) can raise serum PSA levels [108]. Thus, more effective biomarkers are needed. Differential display 3 (DD3), also known as prostate cancer antigen 3 (PCA3), is an lncRNA that was shown to be significantly overexpressed in PCa [109]. It has since become an FDA approved biomarker that has higher specificity than PSA [110].
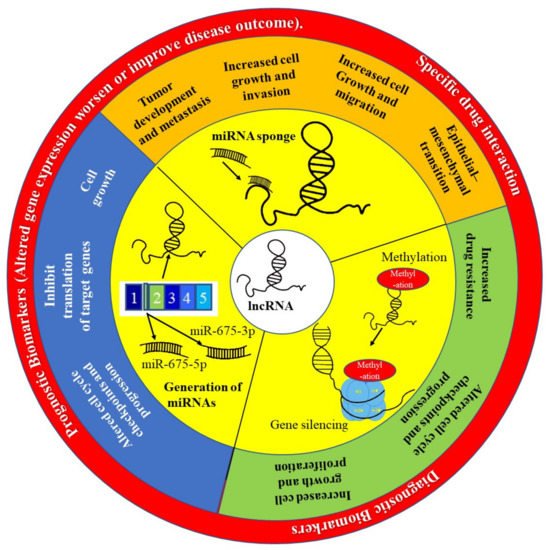
Figure 5. The role of lncRNA in precision oncology. The lncRNA H19 has been implicated as playing an important role in the development and progression of many cancers, including PCa. This lncRNA is able to act by preventing many miRNAs from performing their function by acting like a sponge and binding to these miRNAs preventing them from targeting MRNAs. The H19 gene is also spliced to generate miRNAs such as miR-675-3p and miR-675-5p. Finally, H19 can silence gene expression through methylation of histones.
In endometrial cancer, the lncRNA H19 has been shown to be significantly overexpressed in 60% of EC [111]. Its expression levels are known to increase with the progression of tumor grade. In a study by Peng et al. [112], it was shown that upregulation of H19 is linked to poor prognosis in The Cancer Genome Atlas (TCGA) datasets. This suggests that H19 may be a suitable diagnostics biomarker and a potential therapeutic target for endometrial cancer [107].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020628
This entry is offline, you can click here to edit this entry!
