Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
A few centuries ago, the first vaccine vial was formulated, and since then, they have resulted in an eminent reduction in infectious diseases associated morbidity and mortality. The discovery of the novel SARS-CoV-2 virus and the COVID-19 disease and its steady progression to a global pandemic with 603,711,760 confirmed cases and 6,484,136 reported deaths according to the World Health Organization (WHO) on 7 September 2022 was exceedingly catastrophic.
- COVID-19
- SARS-CoV-2
- vaccine
1. Introduction
Towards the end of December 2019, the Coronavirus Disease (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), originated in Wuhan, Hubei province, China [1]. A pandemic was declared by the World Health Organization (WHO) on 11 March 2020, and as of 7 September 2022, a total number of 603,711,760 cases and 6,484,136 casualties were reported worldwide [2]. COVID-19 affected healthcare systems worldwide at every level [2][3]. The disease spectrum of this pathogen ranges from a mild self-limiting infection to potentially fatal disease with inflated morbidity and mortality figures for individuals with coexisting morbidities such as diabetes mellitus, obesity and other lifestyle diseases, along with poorer outcomes in patients with respiratory comorbidities, such as COPD [4][5].
Akin to other RNA viruses, SARS-CoV-2 is also susceptible to genetic variation and mutation, leading to the development of multiple variants. The WHO formulated the Technical Advisory Group on Virus Evolution (previously referred to as WHO Virus Evolution Working Group) to name and characterize the evolving variants of the virus, which have been listed below [6].
2. Variants of Concern (VOC)
2.1. Current VOC
B.1.1.529 lineage—Alias for the Omicron variant; it was initially reported in South Africa at the Lancet Laboratory during the November of 2021. Its molecular structure comprises numerous mutations especially of the spike protein (S) which plays a vital part in improving the infectivity, transmissibility as well as the immune evasion for the individual [7][8].
2.2. Past VOC
B.1.1.7 lineage—Alpha variant or GRY was discovered initially in the United Kingdom in December of 2020. It was shortlisted as a VOC due to the S-gene target failure in PCR samples, as well as the presence of 17 mutations in its genome. Significance of these changes is observed by the markedly raised affinity of the spike protein to ACE-2 receptors augmenting attachment and raising COVID-19 severity as compared to the other varied variants in circulation [9].
B.1.351 lineage—The Beta variant was initially discovered in South Africa in October of 2020 [10]. The viral genome contains nine mutations in spike protein alone. It has an increased transmission risk and reduced success in management with convalescent sera and monoclonal antibody therapy [11].
B.1.1.28.1 lineage—In December 2020, Brazil reported the Gamma variant [12]. It bears ten mutations on spike protein, three of which were rather homogenous to VOC B.1.351. This variant also decreases the reaction to monoclonal antibody therapy and the convalescent sera [11].
B.1.617 lineage—The Delta variant was initially discovered in India in October of 2020. This VOC shows a genetic variation at the receptor binding dominion of the spike protein, which raised the affinity of the virus to bind to ACE2 receptors, providing it with far more advanced transmissibility [12][13].
3. Variants of Interest (VOI)
3.1. Current VOI
At present, there is no VOI in circulation.
3.2. Past VOI
This category includes variants such as Epsilon, Eta, Iota, Kappa, Lambda and many others, which were classified as such because of their potential to show a reduced response to an antibody or vaccine sera management [6].
In a background of aggressive disease and treatment failure, prevention might be the strategy that could successfully redeem stability in global health, the key to which is the rapid development, manufacturing and distribution of vaccines. Since the formulation of the first vaccine vial a few centuries ago, vaccines have progressively led to a remarkable decrease in the morbidity and mortality caused by various infectious diseases [14]. In this vein, as of 12 September 2022, a total of 12,613,484,608 vaccine doses have been administered [2].
Researchers examine the efficacy and safety of various vaccines against COVID-19 made available by a number of organizations worldwide. According to the WHO, in September 2022, 199 vaccine candidates were in the preclinical phase, and 172 vaccines were registered in clinical trials. Among the clinical applicants, 54 vaccines are in phase I (Testing safety and dosing), 15 vaccines in phase II (expanded safety trials), 45 vaccines in phase III (large-scale efficacy testing) and 11 vaccines have been granted Emergency Use Listing (EUL) [15].
4. COVID Vaccines: Types and Mechanism of Action
The various strains emerge due to mutations mostly in the ACE-2 receptor binding site, the receptor binding domain (RBD) and the N-terminal domains. This encouraged the formulation of many vaccines with distinct working principles, mainly divided into four groups.
4.1. mRNA Vaccines
These vaccines use synthetically manufactured mRNA, which infects the host cells and produces a component of S protein. The body then degrades it while the protein triggers the production of antibodies. These antibodies prepare the body to tackle any future infection with minimal risk of adverse effects. The vaccines using this mechanism are Pfizer and Moderna, produced by BioNTech and Sanofi, respectively [16]. BNT162b2 developed by Pfizer/BioNtech elicits an immunological response by inducing IgG, IgA, CD8+ cells, or CD4+ cells, while mRNA-1273 developed by Moderna induces CD8 T cell response [17].
4.2. Viral Vaccines
These are modified versions of a virus belonging to a different genus used as a vector. It interacts with the immune cells and aids them in acknowledging and outwitting the pathogenic virus. Once injected, the immune cells of the body detect the presence of foreign antigen and activate an immune response by producing antibody-producing B cells and T cells that seek out and destroy infected cells. T cells act by examining the storage of proteins expressed on the surfaces of cells. Since they can recognize the body’s own proteins as ‘self’, if they find a foreign protein, they activate an immune response against the cell storing it [18]. However, their use is limited due to the increased risk of side effects. As of April 2021, the vaccines available using this mechanism are the Janssen, AstraZeneca and Sputnik V vaccines produced by Johnson & Johnson, Oxford-AstraZeneca PLC and Panacea Biotec, respectively [19].
4.3. Whole Pathogen Vaccines
One of the most prevalent and age-old established vaccines is currently of two types—live attenuated and inactivated. The inactivated vaccines are prepared by destroying the virus’s genetic material with chemicals, heat and radiation. Since these vaccines are versions of weak natural pathogens, the immune system activates a range of defenses such as killer T cells which identify and destroy infected cells, helper T cells which support antibody production and antibody-producing B cells which will target pathogens [20]. When introduced into the body, they stimulate antibody-mediated responses, which are weak and relatively less long-lived. Thus, they are always administered along with an adjuvant and booster doses are often required. In contrast, the live attenuated vaccines use a weakened form of the virus in the body. When introduced into the body, these viruses can grow and replicate within the body but cannot cause symptomatic disease in the individual [21]. The vaccines using the inactivated mechanism include Covaxin, Sinopharm, Corovac and Sinovac vaccines. The live attenuated counterparts are yet in the trials phase; nevertheless, one such vaccine approved is Covivac [22].
4.4. Protein Subunit Vaccines
These vaccines, unlike whole virus vaccines, use specific parts of the virus-like fragments, antigens, parts of protein or polysaccharides which are incapable of producing any sort of infection to the body [23]. These fragments have pathogen-associated molecular patterns, which are recognized by the pattern recognition receptors, principally the Toll-like receptors. Upon engagement of the above, the intracellular signaling cascade is triggered. This leads to the release of various proinflammatory molecules, which orchestrates the building of adaptive immunity [24]. The absence of such pathogen-associated molecular patterns would lead to a weaker immune response. Hence, they are given adjuvants as the antigens are insufficient to induce long-term immunity. The vaccines using this mechanism are the Sanofi-GSK, Novova and Dynavax.
Figure 1 depicts a timeline of the major COVID vaccines, whereas Figure 2 depicts the mechanism of action of various types of vaccines.
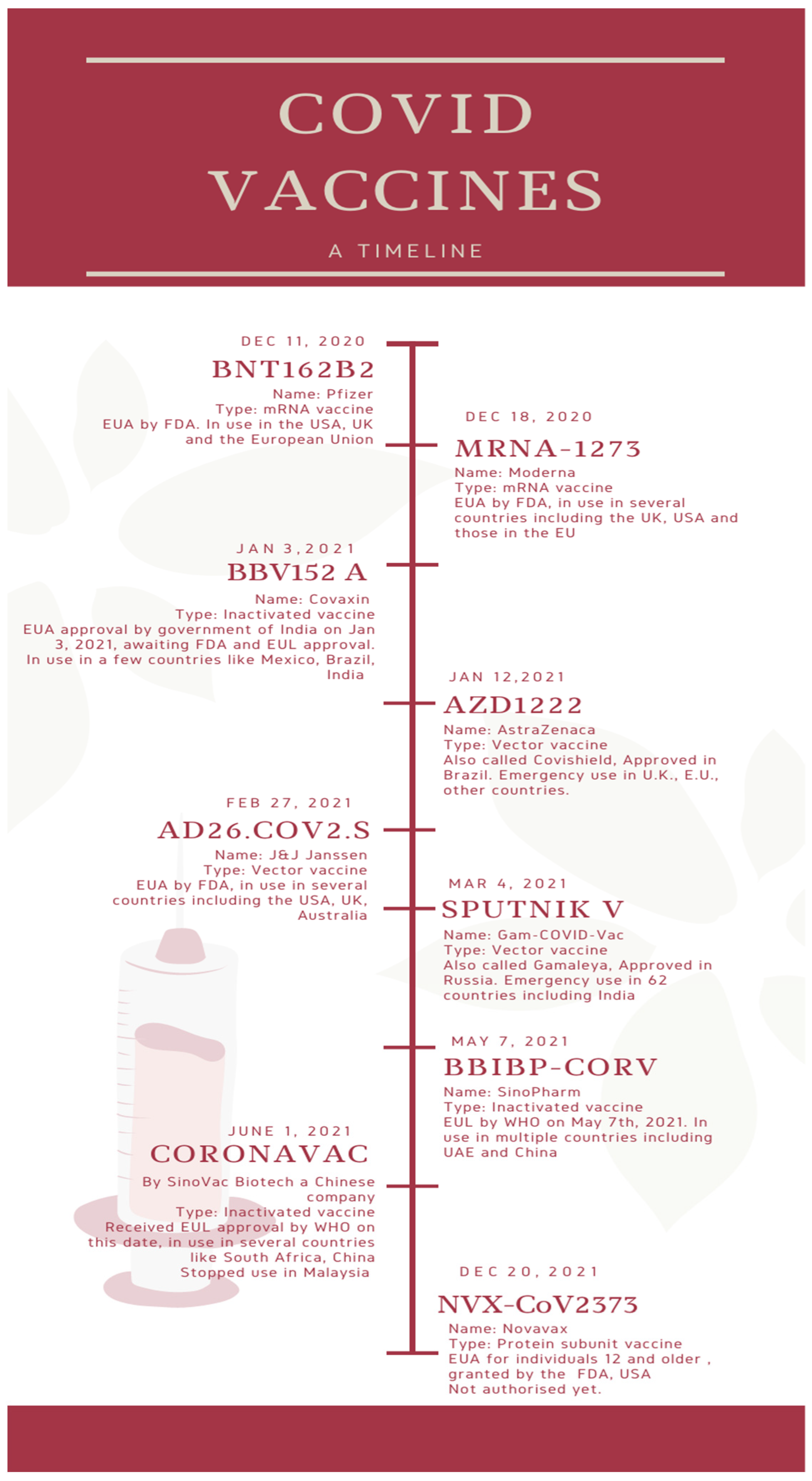
Figure 1. A timeline of the major COVID vaccines.
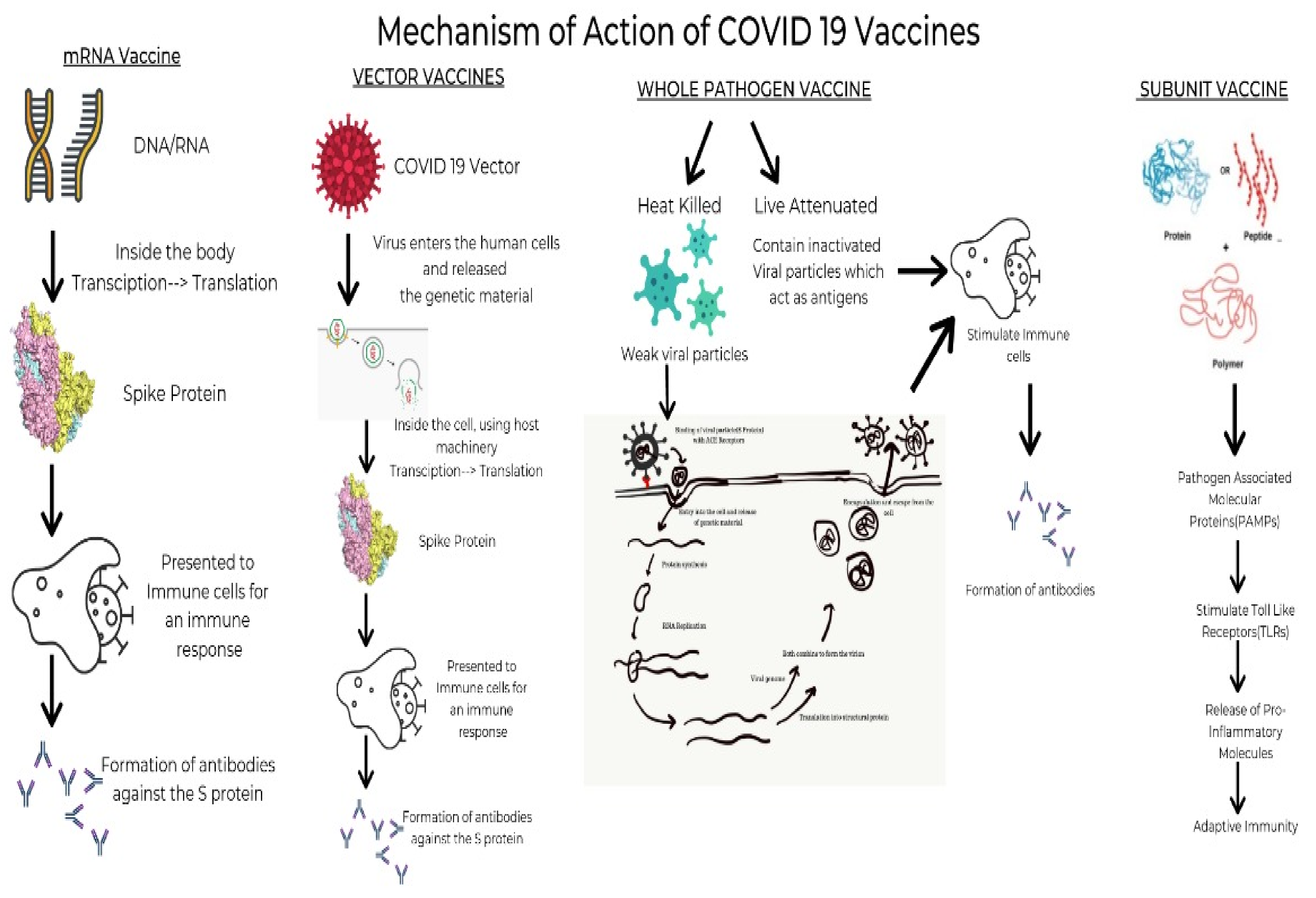
Figure 2. The mechanism of action of various types of vaccines.
5. Efficacy of COVID Vaccines
NPIs (non-pharmaceutical interventions) such as mandatory face masks, national and international travel limitations and marked disinfectant use were somewhat successful in subduing global healthcare downfall, the onus fell upon vaccination strategies to counteract this international threat [26]. While the levels of neutralizing antibodies do not provide a direct measure of vaccine efficacy against the varied variants of SARS-CoV-2 and certainly do not paint the entire picture, barring the effects of T-cell immunity, complement system, it gives an elementary idea of their efficacy in the real world.
5.1. NVX-CoV2373
B.1.1.7 variant—Efficacy trials undertaken by the biotech firm, Novavax revealed that their vaccine was highly effective in producing antibodies for the B.1.1.7 variant of COVID-19 discovered in the UK [27]. Shen et al. compared the B.1.1.7 variant to the D614G in neutralization assays with the serum samples collected from 28 people who received NVX-CoV2373 two weeks after the second dose [28].
B.1.351 variant—Shinde et al. conducted a randomized, double-blind controlled trial among 4387 recipients, which relayed that when participants had been given two doses of the NVX-CoV2373 vaccine, it showed an efficacy of 49.4% (95% (confidence interval) CI, 6.1–72.8) against a COVID-19 infection caused by the variant B.1.351 [29].
B.1.1.529 variant—There is scarce data on the particular effectiveness of NVX-CoV2373 on Omicron. A preprint study reported a decreased response to Omicron compared to Delta and other variants on primary vaccination but cross-reactive antibodies on a three-dose booster regimen [30].
5.2. Ad26.COV2.S
D164G mutation and original Wuhan-Hu-1—Sadoff et al.’s conduction of a randomized, double blind, placebo controlled phase-three trial relayed that Ad26.COV2.S showed immunity against moderate to severe COVID-19, 14 days post dose. In a study population of 19,630 receiving the vaccine, an efficacy of 66.9% (95% CI, 59.0–73.4), which remained 28 days after the dose at around 66.1% (95% CI, 55.0–74.8), was reported. Effectiveness against severe COVID-19 has risen to 76.7% (95% CI, 54.6–89.1) for ≥14 days and 85.4% (95% CI, 54.2–96.9) for ≥28 days [31].
B.1.351 variant—In a trial with 19,630 participants who received the vaccine in South Africa, 94.5% of the sequences were of the B.1.351, and vaccine efficacy sustained a 52.0% in moderate, severe as well as critical conditions of COVID-19 and 73.1% in severe to critical COVID-19 disease ≥14 days after administration. At ≥28 days after administration, efficacy had risen to 64.0% in moderate to severe COVID-19 disease and 81.7% in severe COVID-19 disease. In samples collected in Brazil, 69% showed P.2 lineage carrying the E484K mutation. Despite infection from varied variants, the COVID-19 vaccinations, formulated on the Wuhan-Hu-1 strain, vaccine efficacy remained high. From this, it can be inferred that these vaccines show a cross-protective efficacy with the new variants in SA and Brazil [31].
B.1.1.529—During the Omicron surge, a study was conducted on the population in South Africa in which 162,637 PCR tests were analyzed, of which 93,854 (57.7%) were taken from recipients of both the doses of the BNT162b2 vaccine which were administered forty-two days apart from each other or two doses of the Ad26.COV2.S vaccine which were administered four to six months apart from each other. Within this fraction, of the 34% that were positive, 1.6% underwent admission to a hospital and 0.5% were critical with ICU admission. Of the recipients of the Ad26.COV2.S vaccine, immunity against hospitalization for the disease showed 55% (95% CI, 22–74) after the second dose in less than 13 days, 74% (95% CI, 57–84) at 14 to 27 days, and 72% (95% CI, 59–81) at 1 to 2 months; protection against ICU admission was 69% (95% CI, 26–87) at 14 to 27 days and 82% (95% CI, 57–93) at one to two months post the administration of the second dose [32].
5.3. BNT162b2
D164G mutation, original Wuhan-Hu-1, B.1.1.7, B.1.351—Tada et al. took sera sampling from people who underwent vaccination with BNT162b2 and analyzed their neutralizing activity against D164G mutation strain, B.1.1.7 lineage and B.1.351 lineage spike proteins. Serum samples of vaccinated individuals neutralized D164G strain with seven-fold raised antibody titer than convalescent serum [33]. Sera which neutralized the virus with B.1.1.7 spike protein showed equivalent results, which relayed that the vaccine provides a raised immunity against this variant. There was a threefold reduction in the titer when the sera neutralized the virus with B.1.351 spike protein. This can be credited to the E484K mutation present. Correspondingly, a study using a B.1.1.7 pseudo virus showed the vaccine to remain effective against variants with a slight decrease in neutralization [34]. A study conducted in Qatar, a case–control study of 265,410 persons having received the two-dose regimen, relayed that the vaccine’s efficacy against the B.1.1.7 variant was 89.5% (95% CI, 85.9–92.3) <14 days post the 2nd dose. The efficacy against the B.1.351 variant was 75.0% (95% CI, 70.5–78.9) [35].
P.1 lineage—Dejnirattisai et al. studied the antibody evasion of the P.1 strain. Sera from 25 BNT162b2 vaccinated individuals were used. They concluded that the neutralization titers against P.1 strain were similar to that of B.1.1.7, thus inferring adequate cross-protection of vaccinated individuals against it. Another observation was that B.1.351, or the South African Variant, had a maximum reduction in titer [36].
B.1.617.1 and B.1.617.2 lineage—Liu C et al. compared the reduction in neutralization of variants B.1.617.1&2. There was a reduction of 2.7-fold in the sera of 20 BNT162b2 vaccinated individuals for B.1.617.1 and a 2.5-fold decrease for B.1.617.2. These reductions were similar to those of B.1.1.7 and P.1, indicating that there is no sizable escape in neutralization, unlike the reduction seen in B.1.351 [37]. In a similar neutralization assay study, Liu J et al. reported a modest neutralization reduction when compared to wild-type virus of B.1.617 lineage, the reduction more powerful in B.1.617.1 strain. Although reduction was noted, BNT162b2 immune sera still competently neutralized all strains of this lineage [38]. Zani A et al. studied the neutralization of B.1.525 lineage in addition to other variants. They reported that the virus of said lineage was sufficiently neutralized by the sera of 37 BNT162b2 vaccinated people, as compared neutralization of B.1.1.7 and D164G mutation lineage [39].
5.4. BBV152A
B.1.1.7—The neutralization assay study by Sapkal et al. using sera of individuals vaccinated with BBV152A showed that the escape of the UK variant from this vaccine is unlikely [40].
B.1.351 and B.1.617.2—Yadav et al., in their study of neutralization of B.1.351 and B.1.617.2 variants with the serum of 20 recipients of BBV152 A, reported reduction in titers with these variants, but demonstrated that the neutralizing potential of the vaccine remains well established [41].
B.1.1.28.2—The results of yet another study conducted by Sapkal et al. revealed a 1.92-fold reduction in neutralization titers, when compared to D164G mutation lineage [42].
B.1.1.529—In a study that explored vaccine efficacy against this VOC, virus shedding and lung viral load, along with less morbid disease were relayed in the vaccinated cohorts as compared to the placebo groups. Presently, it is found that a COVAXIN® booster dose augments efficacy of the vaccine against the Delta COVID-19 disease and also offers immunity against morbidity caused by the Omicron variant [43].
5.5. mRNA-1273
B.1.1.7—In a study in Qatar, with 181,304 recipients of the full two-dose regimen, the calculated effectiveness against infection with B.1.1.7 was scarce for the initial two weeks after the first dose, which rose markedly to 81.6% (95% CI, 73.1–87.8%) in the 3rd and 94.4% (95% CI, 89.1–97.5%) in the 4th week and attained 99.2% (95% CI, 95.3–100.0%) in the 2nd week after the subsequent 2nd dose, whereas it was 100% (95% CI, 91.8–100.0%) post 14 days of the second dose [44].
B.1.351—In the above-mentioned study, PCR positive samples of the B.1.351 variant was also collected, and the effectiveness against COVID 19 with B.1.351 was scarce for the initial 2 weeks after the initial dose but markedly raised at the 3rd week to acquire a 47.9% (95% CI, 39.5–55.2%). Efficacy was 73.7% (95% CI, 67.6–78.8%) in the 4th week prior to the 2nd dose and attained 96.4% (95% CI, 94.3–97.9%) in the 2nd week post the 2nd dose and 96.4% (95% CI, 91.9–98.7%) post 14 days of the second dose [44].
B.1.1.529—As one would expect, any strain showing minor changes in spike proteins showed increased escape from humoral response induced by primary (two dose) vaccination. Omicron proved to be no different, due to its highly mutated spike proteins, it escaped neutralization in a large proportion of people who had received two doses of mRNA-1273. Interestingly though, people who had received a booster in the three months prior to a study conducted by Garcia-Beltran WF et al., there were cross neutralization responses to Omicron, thus indicating that a booster of mRNA-1273 increased protection against this VOC mRNA-1273 and BNT162b2 vaccines offer a significantly better response than the previously established J&J vaccines [45][46].
5.6. AZD-1222
B.1.1.7—The vaccine showed reduced titers for neutralization with this lineage, but clinical efficacy against features of the disease caused by variant B.1.1.7 was adequate, suggesting that low neutralizing antibody titers are adequate to provide protection [47].
B.1.351—A randomized control trial, with 2026 participants, conducted in Africa concluded that 2 doses of this vaccine had no effect against mild to moderate disease caused by B.1.351, but notably there were no reports of morbidity from severe disease either. This reduced efficacy should be considered, with the background that the first dose of this vaccine had 75% efficacy (95% CI, 8.7 to 95.5) in protecting against mild to moderate infection of COVID-19 before the emergence of this variant of concern [48].
B.1.617—The geometric mean neutralization titers against B.1.617.1 were 2.7 times less when compared to the Victoria virus for the Pfizer-BioNTech vaccine serum and 2.6 times less for the Oxford-AstraZeneca vaccine. The reductions were commensurable in scale with those seen with B.1.1.7 and P.1, with no indication of broad abdication from neutralization, contrary to what is seen with B.1.351. From this, researchers can conclude that the current vaccine is sufficient to provide protection against severe infections caused by the B.1.617 variant, although parallelly an increase in breakthrough infections can be expected [37].
B.1.1.529—In a retrospective analysis, 886,774 Omicron-infected individuals were identified and effectiveness studied; it was found that after two doses of the AZD-1222 vaccine, no effect was seen. The efficacy after an AZD-1222 primary course had increased to 70.1% (95% CI, 69.5–70.7) after 2–4 weeks of an mRNA-1273 booster and reduced to 60.9% (95% CI, 59.7–62.1) after 5–9 weeks [45].
5.7. Sputnik V
The provisional outcomes of the past 3 Gram-COVID-Vac trials, in which 19,866 received two doses of the vaccine, exhibit that the vaccine is 96.1% (95% CI, 85.6–95.2) effective against disease caused by SARS-CoV2. This includes the period 21 days after the 1st dose to the day of receiving the 2nd dose. However, unlike some other vaccine candidates, the outcomes of the vaccine were not 100% (95% CI, 94.4–100) effective against the severe form of COVID-19; The results were preliminary as this was a secondary outcome [49].
5.8. CoronVac
In a double-blind, placebo-controlled, randomized phase I/II clinical trial by Zhang Y et al., examining the safety and efficacy of CoronaVac on healthy adults aged 18–59 (743 participants received at least one dose), it was reported that CoronaVac was well tolerated and induced adequate immune response against a COVID-19 infection. According to the trial, protective efficacy is yet to be determined. The studies on the efficacy of the vaccine against different strains are lacking [50].
5.9. Sinopharm
According to Huang et al., the 501Y.V2 variant remains under the umbrella of protection offered by vaccines targeting the whole virus (BBIBP-CorV). The potential 1.5–1.6 times reduction in neutralizing GMTs must be considered for their effect on the clinical efficacy of these vaccines. For these vaccines, immune serum samples neutralize both variants 501Y.V2 and D614G [51].
Two case studies from Brazil reported breakthrough infection in two vaccines: in one, 122 days following administration of the second dose, and in the other, 106 days post administration of the second dose. Both were reported to be infected by the P.1 variant of SARS-CoV2. Both patients recovered fully and did not develop sequelae or severe illness [52].
This entry is adapted from the peer-reviewed paper 10.3390/diseases10040112
References
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules 2020, 26, 39.
- WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 9 September 2022).
- Revythis, A.; Limbu, A.; Mikropoulos, C.; Ghose, A.; Sanchez, E.; Sheriff, M.; Boussios, S. The Experience of a Single NHS England Trust on the Impact of the COVID-19 Pandemic on Junior and Middle-Grade Doctors: What Is Next? Int. J. Environ. Res. Public Health 2021, 18, 10413.
- Russell, B.; Moss, C.L.; Shah, V.; Ko, T.K.; Palmer, K.; Sylva, R.; George, G.; Monroy-Iglesias, M.J.; Patten, P.; Ceesay, M.M.; et al. Risk of COVID-19 death in cancer patients: An analysis from Guy’s Cancer Centre and King’s College Hospital in London. Br. J. Cancer 2021, 125, 939–947.
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076.
- Tracking SARS-CoV-2 Variants. 2022. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 9 September 2022).
- Kannan, S.; Shaik Syed Ali, P.; Sheeza, A. Omicron (B.1.1.529)-variant of concern-molecular profile and epidemiology: A mini review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 8019–8022.
- Ren, S.Y.; Wang, W.B.; Gao, R.D.; Zhou, A.M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 2022, 10, 1–11.
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.M. Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443.
- Wang, P.; Casner, R.G.; Nair, M.S.; Wang, M.; Yu, J.; Cerutti, G.; Liu, L.; Kwong, P.D.; Huang, Y.; Shapiro, L.; et al. Increased Resistance of SARS-CoV-2 Variant P.1 to Antibody Neutralization. Cell Host Microbe 2021, 29, 747–751.e4.
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542.
- Dholariya, S.; Parchwani, D.N.; Singh, R.; Sonagra, A.; Motiani, A.; Patel, D. Notable and Emerging Variants of SARS-CoV-2 Virus: A Quick Glance. Indian J. Clin. Biochem. 2021, 36, 451–458.
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963.
- COVID19 Vaccine Tracker. 2022. Available online: https://covid19.trackvaccines.org/ (accessed on 9 September 2022).
- Understanding mRNA COVID-19 Vaccines. Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html (accessed on 13 October 2021).
- Goyal, K.; Goel, H.; Baranwal, P.; Tewary, A.; Dixit, A.; Pandey, A.K.; Benjamin, M.; Tanwar, P.; Dey, A.; Khan, F.; et al. Immunological Mechanisms of Vaccine-Induced Protection against SARS-CoV-2 in Humans. Immuno 2021, 1, 442–456.
- How do vector vaccines work? WHO Collaborating Centre for Vaccine Safety. 2021. Available online: https://www.covid19infovaccines.com/en-posts/how-do-vector-vaccines-work (accessed on 12 November 2022).
- Understanding Viral Vector COVID-19 Vaccines. Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/viralvector.html (accessed on 13 October 2021).
- How do inactivated vaccines work? WHO Collaborating Centre for Vaccine Safety. 2021. Available online: https://www.covid19infovaccines.com/en-posts/how-do-inactivated-vaccines-work (accessed on 12 November 2022).
- Vaccine Types. National Institute of Allergy and Infectious Diseases. 2021. Available online: https://www.niaid.nih.gov/research/vaccine-types (accessed on 13 October 2021).
- Shahzamani, K.; Mahmoudian, F.; Ahangarzadeh, S.; Ranjbar, M.M.; Beikmohammadi, L.; Bahrami, S.; Mohammadi, E.; Esfandyari, S.; Alibakhshi, A.; Javanmard, S.H. Vaccine design and delivery approaches for COVID-19. Int. Immunopharmacol. 2021, 100, 108086.
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777.
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273.
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636.
- El-Elimat, T.; AbuAlSamen, M.M.; Almomani, B.A.; Al-Sawalha, N.A.; Alali, F.Q. Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. PLoS ONE 2021, 16, e0250555.
- Callaway, E.; Mallapaty, S. Novavax offers first evidence that COVID vaccines protect people against variants. Nature 2021, 590, 17.
- Shen, X.; Tang, H.; McDanal, C.; Wagh, K.; Fischer, W.; Theiler, J.; Yoon, H.; Li, D.; Haynes, B.F.; Sanders, K.O.; et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe 2021, 29, 529–539.e3.
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909.
- Bhiman, J.N.; Richardson, S.I.; Lambson, B.E.; Kgagudi, P.; Mzindle, N.; Kaldine, H.; Crowther, C.; Gray, G.; Bekker, L.G.; Shinde, V.; et al. Novavax NVX-COV2373 triggers potent neutralization of Omicron sub-lineages. bioRxiv 2022.
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201.
- Gray, G.; Collie, S.; Goga, A.; Garrett, N.; Champion, J.; Seocharan, I.; Bamford, L.; Moultrie, H.; Bekker, L.G. Effectiveness of Ad26.COV2.S and BNT162b2 Vaccines against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 2243–2245.
- Tada, T.; Dcosta, B.M.; Samanovic-Golden, M.; Herati, R.S.; Cornelius, A.; Mulligan, M.J.; Landau, N.R. Neutralization of viruses with European, South African, and United States SARS-CoV-2 variant spike proteins by convalescent sera and BNT162b2 mRNA vaccine-elicited antibodies. bioRxiv 2021.
- Muik, A.; Wallisch, A.K.; Sänger, B.; Swanson, K.A.; Mühl, J.; Chen, W.; Cai, H.; Maurus, D.; Sarkar, R.; Türeci, Ö.; et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 2021, 371, 1152–1153.
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A.; National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189.
- Dejnirattisai, W.; Zhou, D.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell 2021, 184, 2939–2954.e9.
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y.; et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.e13.
- Liu, J.; Liu, Y.; Xia, H.; Zou, J.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Cutler, M.; Cooper, D.; Muik, A.; et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature 2021, 596, 273–275.
- Zani, A.; Caccuri, F.; Messali, S.; Bonfanti, C.; Caruso, A. Serosurvey in BNT162b2 vaccine-elicited neutralizing antibodies against authentic B.1, B.1.1.7, B.1.351, B.1.525 and P.1 SARS-CoV-2 variants. Emerg. Microbes Infect. 2021, 10, 1241–1243.
- Sapkal, G.; Yadav, P.; Ella, R.; Deshpande, G.R.; Sahay, R.R.; Gupta, N.; Mohan, V.K.; Abraham, P.; Panda, S.; Bhargava, B. Neutralization of UK-variant VUI 202012/01 with COVAXIN vaccinated human serum. bioRxiv 2021.
- Yadav, P.D.; Sapkal, G.; Ella, R.; Sahay, R.R.; Nyayanit, D.A.; Patil, D.Y.; Deshpande, G.; Shete, A.M.; Gupta, N.; Mohanet, V.K.; et al. Neutralization against B.1.351 and B.1.617.2 with sera of COVID-19 recovered cases and vaccines of BBV152. bioRxiv 2021.
- Sapkal, G.; Yadav, P.D.; Ella, R.; Abraham, P.; Patil, D.Y.; Gupta, N.; Panda, S.; Mohan, V.K.; Bhargava, B. Neutralization of VUI B.1.1.28 P2 variant with sera of COVID-19 recovered cases and recipients of Covaxin an inactivated COVID-19 vaccine. J. Travel. Med. 2021, 28, taab077.
- Yadav, P.D.; Mohandas, S.; Shete, A.; Sapkal, G.; Deshpande, G.; Kumar, A.; Wakchaure, K.; Dighe, H.; Jain, R.; Ganneru, B.; et al. Protective efficacy of COVAXIN® against Delta and Omicron variants in hamster model. iScience. 2022, 25, 105178.
- Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Hasan, M.R.; Malek, J.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021, 27, 1614–1621.
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546.
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2021, 185, 457–466.e4.
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomized controlled trial. Lancet 2021, 397, 1351–1362.
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898.
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681.
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192.
- Huang, B.; Dai, L.; Wang, H.; Hu, Z.; Yang, X.; Tan, W.; Gao, G.F. Neutralization of SARS-CoV-2 VOC 501Y.V2 by human antisera elicited by both inactivated BBIBP-CorV and recombinant dimeric RBD ZF2001 vaccines. bioRxiv 2021.
- Estofolete, C.F.; Banho, C.A.; Campos, G.R.F.; Marques, B.C.; Sacchetto, L.; Ullmann, L.S.; Possebon, F.S.; Machado, L.F.; Syrio, J.D.; Araújo Junior, J.P.; et al. Case Study of Two Post Vaccination SARS-CoV-2 Infections with P1 Variants in CoronaVac Vaccines in Brazil. Viruses 2021, 13, 1237.
This entry is offline, you can click here to edit this entry!
