Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Neural injuries affect millions globally, significantly impacting their quality of life. The inability of these injuries to heal, limited ability to regenerate, and the lack of available treatments make regenerative medicine and tissue engineering a promising field of research for developing methods for nerve repair.
- nerve tissue engineering
- biocompatibility
- polymers
- biomaterials
- nanomaterials
1. Introduction
The nervous system is divided into two distinct components: the central nervous system (CNS), composed of the brain and the spinal cord, and the peripheral nervous system (PNS), composed of the cranial and spinal nerves that extend from the CNS throughout the body. The normal, healthy nervous system is responsible for sensory, motor, and cognitive functions [1]. Neural injuries and diseases disrupt the signaling pathways responsible for these everyday acts, ultimately declining an individual’s quality of life. Affecting millions of people in the United States (US) alone, neural injuries include both CNS injuries, such as traumatic brain injuries (TBIs), spinal cord injuries (SCIs), and neurodegenerative diseases (NDs), as well as peripheral nerve injuries and degeneration (PNI) [2][3][4][5][6]. Each injury has a classification system identifying the nerve damage level [7][8][9]. In the PNS, a lower degree of injury will typically regenerate independently. However, more severe injuries in the PNS and most injuries in the CNS cannot heal without assistance.
A severe PNI is one in which, in addition to the axon and myelin sheath, the tissues surrounding the various bundles of nerve cells (endoneurium, perineurium, and epineurium) are also damaged, and the size of the PNI gap is more significant than 10 mm. Once the endoneurium (the innermost tissue layer) is affected, the injury worsens when the elasticity causes the proximal and distal nerve ends to retract. Widening the gap makes it more difficult for the developing axon to align accurately and reinnervate the target tissue to restore nerve function. The degenerative process that fragments the cytoskeleton of the axon causes cellular debris to accumulate in the affected area alongside the damaged cells from the initial injury. Secreted pro-inflammatory cytokines, immune cells, and harmful reactive oxygen intermediates (ROIs) in the environment further impair the regenerative ability.
While macrophages and Schwann cells help clear debris and direct cell growth in the PNS, their lack of involvement in the brain and spinal cord damages their capacity to regenerate. Like severe PNIs, most CNS injuries result in a sustained inflammatory state filled with cellular debris and impaired by scar tissue. Astrocytes, a supportive glial cell, propagate neuroinflammation and aggregate to form a glial scar preventing self-renewal. The CNS also involves the blood–brain barrier (BBB), whose role is to prevent cells, proteins, and large molecules circulating in the bloodstream from entering the CNS to protect these tissues from harm and shield the sensitive ecosystem. While some CNS injuries damage the BBB with initial insult, others cause secondary injury from the prolonged presence of inflammatory factors that exacerbate cell death, which may occur minutes to months after the original trauma. This disruption allows the peripheral immune cells and exogenous proteins to intrude into the already hostile environment where they are generally absent, further destabilizing the area [10][11][12][13].
Ultimately, both of these processes have the same result, a microenvironment unsuitable for regeneration, perpetuating disruption of regular nervous system functions [14]. Available treatments include various surgical and non-surgical options, though all are limited in restoring full neural function. The gold standard for a PNI is the use of autografts. Clinical use of autografts fails to restore full function in up to 33% of patients and includes the risk associated with the harvest surgery, loss of innervation, scarring and neuroma formation at the donor site, and limited tissue availability [7][10][15].
As a result of the limited ability of peripheral nerves to regenerate without assistance and the lack of availability of efficient therapies for nerve repair, tissue engineering and regenerative medicine have become promising areas of study to develop novel, effective treatments. The goal of tissue engineering and regenerative medicine is to use exogenous cells, biomaterials, and/or additional biological factors to promote a more favorable microenvironment after an injury or disease so that the tissue can be repaired, and function restored. Although reaching this goal is complicated by the intricacies of the nervous system, optimal treatment for neural applications would be: (1) tissues obtained from a source that is easy and ethical to access; (2) therapeutic materials that can promote differentiation into specific neural and glial cell types; (3) a method of administration that is safe, minimally invasive, and causes no short-term or long-term adverse effects; and (4) an effective treatment that fully and consistently restores function [13][16][17] (Figure 1).
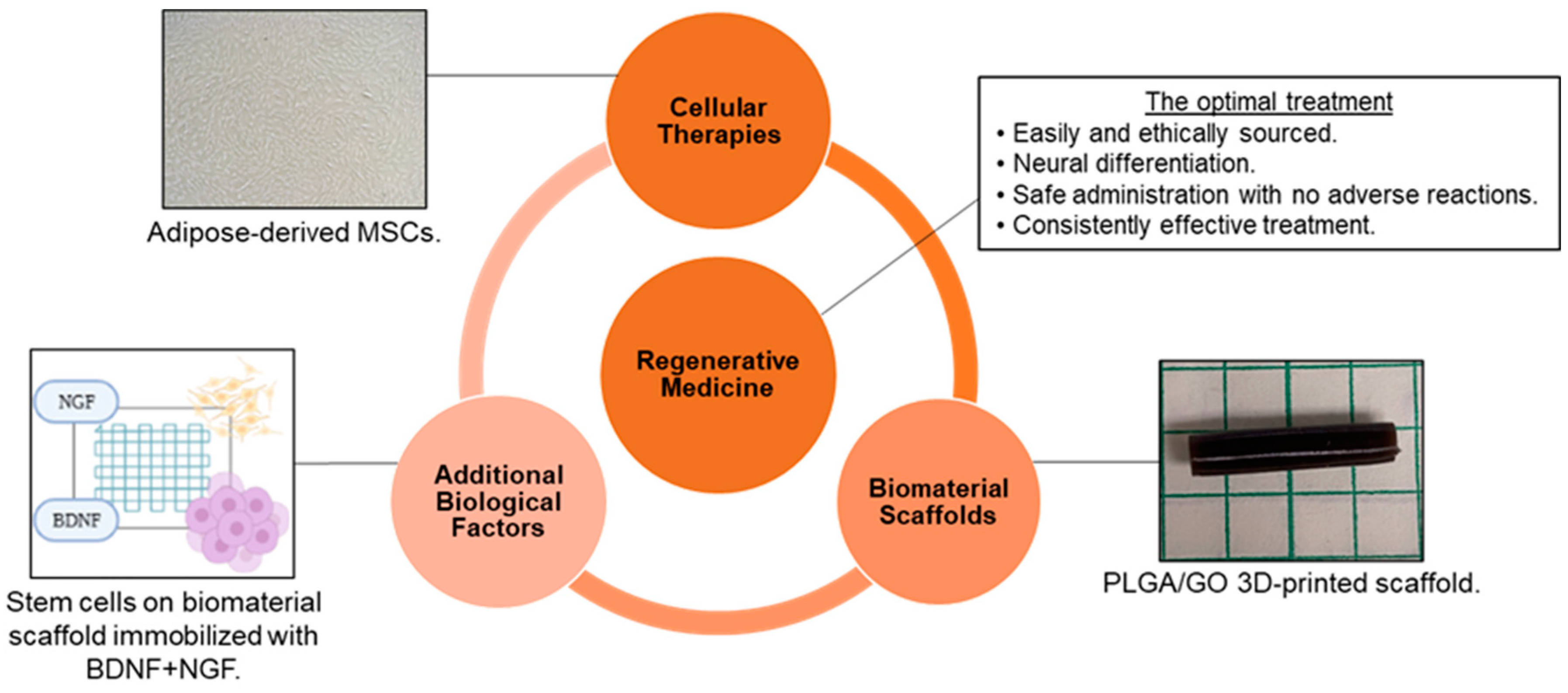
Figure 1. Tissue engineering and regenerative medicine can provide conditions that support physical and functional nerve regeneration. The influence of material and design on cell interactions must be considered to generate the optimal treatment [Images from Harley-Troxell, Dhar, unpublished data; figure made with BioRender and Microsoft PowerPoint].
2. Biocompatibility Assessment
The term biocompatibility is defined by D.F. Williams as “the ability of a material to perform with an appropriate host response in a specific application” [18]. The science community has discussed throughout the conception of biomaterials as to how to characterize biocompatibility of a material and to what degree. Through decades of research in both in vitro and in vivo models, the term can be subdivided into four individual categories: (1) toxicology, (2) reaction to extrinsic microorganisms, (3) mechanical effect, and (4) cell–biomaterial interactions (Figure 2) [19]. This has led to the development of both in vitro and in vivo methods for the assessment of each subdivision, and the expansion of standardized procedures. These methods must answer with high certainty the public concern that a device or material will improve and ensure public safety, taking into consideration the type of biomaterial and biological components being assessed for cytotoxicity. This will determine any potential toxic mechanisms or material property effects that may occur due to reactions with interfering assay reagents, in order to prevent null or false readings. Most of these procedures are standardized by international agencies such as the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM), while other agencies will regulate or enforce these standards, such as the United States Food and Drug Administration (FDA) [20][21][22].
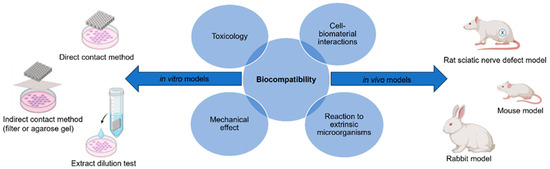
Figure 2. Biocompatibility can be evaluated using the appropriate in vitro and in vivo models to ensure there is no toxicology, mechanical effects, reaction to extrinsic microorganisms, or cell–biomaterial interactions [figure made with BioRender and Microsoft PowerPoint].
3. Polymers for Neural Applications
Numerous materials are available for biological applications in nervous tissues. Arguably, the most important characteristics are those that influence safety and efficacy. For a material to be used in the body, it must be deemed safe, biocompatible, and approved for use by the FDA. The FDA has an established set of guidelines that states that the biocompatibility of each material component should be understood at each step in its formation. This includes the original material before and after polymerization, as well as after sterilization, coatings, and the degradation products. Ultimately, the material should not result in an adverse biological response from the body [23]. A variety of materials have been established as biocompatible, such as the synthetic polymers polyglycolic acid (PGA) and polylactic acid (PLA), which are widely used in biodegradable sutures [24]. Others are more problematic, such as the synthetic polytetrafluorethylene (ePTFE), whose lack of degradability has resulted in long-term foreign body reactions [25]. All polymers have advantages and disadvantages which may differ among natural and synthetic polymers.
3.1. Natural Polymers
Characteristics of natural polymers should include biocompatibility and biodegradability, minimal immunogenicity, and, ideally, provide properties that mimic the biological extracellular matrix (ECM) to provide structural and functional support. Replicating these properties allows for high-quality cellular interactions, including cell adhesion, proliferation, and differentiation for tissue regeneration. However, individual natural polymers tend to be less stable, have poor mechanical properties, and the purification method is less standardized. The variation between polymers and among different products of the same polymer is a potential limitation to translating natural polymers to commercial use because reproducibility is a critical factor in developing an optimal treatment. [13][25][26][27][28][29].
Collagen is an abundant natural polymer found in connective tissue and the ECM and is biocompatible, biodegradable, and bioactive. Currently, five FDA-approved collagen nerve conduits are commercially available. Many in vitro studies, in vivo studies, and clinical trials have identified the effective use of collagen support for wound healing, cell adhesion, migration, proliferation, and functional nerve regeneration. Additionally, collagen has been shown to bridge neural injury gaps up to 20 mm in length [11][25][30]. Collagen has tunable physical characteristics that can aid in directing axonal growth. However, collagen has limited mechanical stress resistance, which can be reinforced with additional biomaterials and/or fabricated into a hydrogel. Fabrication can be difficult due to collagen’s poor manipulability [27][28]. In some cases, collagen has decreased neuroma formation and scarring, but has not wholly eliminated these complications [28][31].
Hyaluronic acid (HA) is a natural polymer found in the ECM of neuroepithelial-derived tissues. In addition to its biocompatibility, HA is known for reproducible outcomes and efficacy in decreasing scar tissue formation [28][31][32]. HA hydrogels are known to be cytocompatible with no significant immune response when co-cultured with human peripheral blood mononuclear cells in vitro [33]. However, its fast degradation and poor mechanical properties limit its solo use. Fortunately, HA can be combined with additional material to optimize its mechanical properties for neural applications [28][31][32].
Silk fibroin is a polypeptide isolated from the natural polymer silk. It is a strong, stable, biodegradable, and biocompatible material. It has also been shown to support cell attachment and neural and Schwann cell proliferation to promote myelinated axon regeneration. Silk fibroin has desirable elasticity and flexibility to avoid collapse, and effective permeability allowing for nutrient exchange. However, silk fibroin’s limitations result from its mechanically weak and fragile properties [28]. Silk fibroin can be processed using several different methods, including 3D bioprinting [25][26][27][34].
Fibrin is a natural polymer whose role in the body involves blood clotting. Fibrin has been shown to improve axonal regeneration and support blood vessel reparation [31]. Fibrin promotes cell adhesion for improved viability, proliferation, and differentiation of neural cells. Fibrin has been shown to prevent fibrous tissue formation and scarring while retaining biocompatibility [28]. Cell viability and neural differentiation have recently been reported after 3D printing (3DP) fibrin-based bioinks containing stem cells.
Chitin is the second most abundant natural polymer on Earth [30]. However, due to its poor solubility, it is often modified to chitosan, maintaining the same chemical structure while adding necessary free amine groups [35]. Chitosan is low-cost, easy to produce, and biocompatible [36]. Although chitosan does not have desirable degradation and mechanical properties, chitosan has ECM-like features that support numerous studies showing significant improvements in functional axon regeneration, cell adhesion, and neural differentiation while decreasing scar formation in vivo [25][26][27][28][30][31][34][35].
3.2. Synthetic Polymers
Many synthetic polymers exhibit biocompatibility without cytotoxicity and can either be biodegradable (also known as bioresorbable), often preferred, or non-degradable. Poor degradation of synthetic polymers limits their use due to the higher potential for chronic inflammation [29]. These polymers also have uniquely beneficial characteristics, including tunability in mechanical characteristics and degradation rate. These polymers are easier to manufacture into complex architectures to better achieve biomimicry of the desired microenvironment, and are readily reproducible. One disadvantage is that these materials can be costlier and more time-consuming to produce. The most common synthetic polymers that support axon regeneration, guidance, and cell migration for improved nerve repair will be discussed [25][26][27][28][34][37].
Silicone and ePTFE are synthetic, non-degradable polymers that successfully treat PNIs. A second surgery to remove the implant may be required if complications, such as long-term foreign body reactions, occur. Despite this shortcoming, these polymers remain biocompatible for PNI healing and successful regeneration of myelinated axons [11][25][30].
Polyesters include PGA, PLA, poly (lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and poly(L-lactide-co-ε-caprolactone) (PLCL). These materials are favorable for use because of biocompatibility, tunable degradability, and limited cytotoxicity of degradation by-products. However, if the degradation occurs too rapidly, an acidic, inflammatory environment has been documented, resulting in tissue necrosis. Interestingly, PLGA’s degradation can be adjusted by changing the molar ratio of PGA to PLA, while PLCL’s copolymerization can neutralize the acidic environment [25][30][37][38][39]. Polyesters have desirable mechanical properties, flexibility, porosity, stability, solubility, hydrophilicity, crystallinity, and processability, making them valuable candidates for bioinks and 3DP methods. Yet, evidence of material deformation has been observed with long-term strain [16].
Polyethylene glycol (PEG) is a biocompatible, synthetic polymer often used to adjust hydrogel swelling and mechanical properties [29][34][37]. PEG is often modified before use due to its easy processability, allowing for chemical modification and cross-linking, as well as its use in manufacturing methods such as 3DP [26]. PEG biodegrades poorly, limiting its use in vivo [34][40]. However, when modified, PEG can improve the microenvironment and support neural cell adhesion, proliferation, and differentiation [31].
Finally, polyurethane (PU) is a versatile synthetic polymer with adjustable mechanical and degradation properties. The Advincula group has reported PU/graphene nanocomposites to have potential for biomedical applications based on relatively stable and low toxicity when co-cultured with mammalian NIH-3T3 cells [41]. However, degradation by-products exhibit potential cytotoxicity [42].
4. Cells That Interact with Biomaterials
Regardless of the material, it is essential to consider the types of cells interacting with the surface when fabricating scaffolds. These cell types include any exogenous cells seeded on the construct and the endogenous cells within the injured area. Regenerative medicine for neural applications will often add primary culture Schwann cells, neural stem cells (NSCs), MSCs, embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs) to scaffolds [29][31]. The source of these cells can be autologous, allogeneic, or xenogeneic [16]. Overall, the type of cell and how it will interact with the biomaterial surface influences its ability to repair neural injury. Meanwhile, the body will have systemic and localized reactions to the implanted material. Following the standard pattern of wound healing after a nerve is damaged, the foreign object implanted in the injured area will interact with peripheral immune cells, fibroblasts, progenitor cells, and the neural and glial cells that form the body’s nervous system [13][43][44]. The interactions between these cells and different biomaterials will determine if the entire construct is both biocompatible and capable of promoting functional nerve recovery.
Schwann cells have been studied in conduits developed to treat PNIs. They are effective at directing axon elongation, neurite outgrowth, and forming a new myelin sheath, but difficulties in sourcing and purification have limited their use [16][31]. Stem cells are more commonly studied, having exhibited promotion of repair in neural injuries when added to a biomaterial scaffold. iPSCs seeded onto a PLA/PCL co-polymer conduit accelerated peripheral nerve repair [45]. The iPSCs were pre-differentiated into Schwann cell lineage before implanting the conduit into a sciatic nerve defect mouse model. This process removed the issues with sourcing and purification while retaining the benefits for directing axon growth. They found that adding the iPSCs to their material significantly enhanced their mice’s functional recovery and promoted neural and myelin tissue histologically [45].
When a nerve injury occurs, or a scaffold is implanted, many cell types are recruited endogenously to repair the damage. Although inflammation is a natural response to injury, the harm comes when the inflammation is prolonged. Local proteins adsorb to the material surface, and this material–cell interface’s properties determine the immune response’s extent [46]. If the interactions at the interface are non-immunogenic, the body accepts the material, i.e., it is biocompatible, and the inflammatory response will not last longer than a few days. However, if the material is identified as a foreign body, the secretion of inflammatory cytokines and chemokines will initiate a response from the immune system [46][47][48]. Phagocytic cells, such as neutrophils, monocytes, local macrophages, and microglia, are attracted to these signals and migrate to the injured area [46][47][48][49][50]. These cells will remove cellular debris and pathogens while releasing reactive oxygen species (ROS) that cause additional damage [48]. However, the recruitment of these cells is necessary to help with regeneration, as they also secrete beneficial growth factors that promote tissue organization and angiogenesis [47][48][50]. The monocytes recruited to the area differentiate into macrophages. These macrophages attempt to engulf the scaffold but cannot phagocytize such a significant material and become stressed. The microenvironment becomes chronically inflamed, and the macrophages begin to morph together and form foreign body giant cells (FBGCs). A fibrous tissue encapsulates the material and attempts to expel the material from the body, rejecting the implant [46][49][50]. Although the innate immune cells are the first line of defense, the adaptive T cells and B cells often complement the system, recognizing specific antigens and aiding in the cascade of inflammatory factors [49][50][51]. When the inflammation begins to subside around the biocompatible scaffold, neural and glial progenitor cells will move through the area to regenerate and remodel the tissue. Depending on whether the injury occurs in the CNS or PNS, these cells will differentiate into neural and glial cells, including astrocytes, oligodendrocytes, or Schwann cells, to rebuild the nerve. Each cell type has a vital role in the nervous system that helps regulate neural activity and the exchange of nutrients in a healthy microenvironment. Finally, macrophages, fibroblasts, and endothelial cells will reconstruct the surrounding ECM and vasculature [46].
5. Biomaterial Modifications Influence Cell Behavior
Although these polymers have desirable biocompatibility, tunable degradation, and mechanical properties that benefit biological use, most polymers are not used without additional modifications. These polymers are altered to optimize their use in neural applications while retaining the benefits of their material. Nanomaterials, such as graphene or gold, are commonly incorporated due to their conductive capabilities [36][52][53][54][55][56][57]. Polymers also can be altered for a more specific application. For example, cross-linking polymers to create hydrogels are suitable for use in the CNS, specifically for TBIs, and hollow conduits and wraps are more suitable for use in PNIs and SCIs due to the shape of axons and the spinal cord. While there have been many studies on the effects of polymers, incorporating nanomaterials and cross-linking changes the material’s characterization and the behavior of the cells they interact with [37].
5.1. Hydrogel Formation
Hydrogels can be used for various applications, including nerve tissue engineering. They are often preferred for CNS injuries, particularly TBIs, where nerve guidance conduits would be too invasive. Defined as 3D cross-linked polymers with high water content and tunable properties, hydrogels can comprise individual polymers or composites of two or more materials. When forming a composite, the strengths and weaknesses of the unique polymers are combined [11]. Overall, hydrogels tend to induce minor inflammation, have favorable biocompatibility and biodegradability, but have weak mechanical properties [29][37][58][59]. The molecular weights and the concentrations of the distinct polymers, alongside the cross-linking method, can be used to alter the characteristics of the hydrogel. Standard cross-linking methods include ultraviolet (UV) light, pH, and temperature.
The gelation time is essential because the hydrogel needs to have the appropriate thixotropic properties (injectability, shear-thinning, etc.) to be appropriately processed (3D printed, injected, etc.). A benefit of hydrogels for their use in CNS injuries is that they can fill irregularly shaped injury sites while providing the appropriate structural support required for nerve development [59]. However, without the appropriate gelation, a hydrogel can take hours or days to obtain the proper mechanical stability characteristic of this modification [60].
In addition to the gelation kinetics, the physicochemical attributes of the hydrogel significantly influence cellular behavior at the tissue–material interface. Characteristics such as porosity, micro patterns, single vs. multi-channel, filament diameter, and surface roughness can all change cell adhesion, proliferation, axon guidance, and neural differentiation [28][61]. Hydrogels mimic the natural ECM, providing a microenvironment that allows for cell signaling and cell adhesion to promote nerve regrowth [62]. A porous hydrogel creates the space for increased cell–cell interactions and access to nutrients and supportive molecules [58].
These characteristics of hydrogels, and the physical and chemical cues they provide, are proper modes for delivering cells, drugs, and other biological factors to aid nerve repair. The material encapsulates the factors of choice to support further the environment in which it is implanted [61][62].
5.2. Incorporation of Nanomaterials
Nanomaterials are materials composed of particles that have nanoscale dimensions (<100 µm). These can include various materials, among which carbon-based nanomaterials, such as graphene and its derivatives and gold (Au) nanomaterials, are commonly used. These materials improve polymers’ strength, stiffness, electrical conductivity, topographical features, and surface area while retaining biocompatibility. However, they are not without their challenges. Nanomaterials can improve the loading efficiency, binding efficiency, and sustained release of biomolecules, such as growth factors for enhanced regeneration, or drugs for therapeutic drug delivery. These particles are often conductive and hydrophobic, so they can be challenging to handle as they aggregate together and poorly disperse [27][55][63][64][65].
Graphene has two common functionalizations that often are used for neuroregeneration: graphene oxide (GO) and reduced graphene oxide (rGO). GO has been shown to increase secretions of neurotrophic factors, neural differentiation, cell attachment and proliferation, and neurite outgrowth. rGO offers these same regenerative benefits alongside increased electrical conductivity. Graphene can be incorporated in several ways, including blending with a polymer, or coating a surface [31][34][39][54][65][66].
6. Biomaterial Fabrication Methods
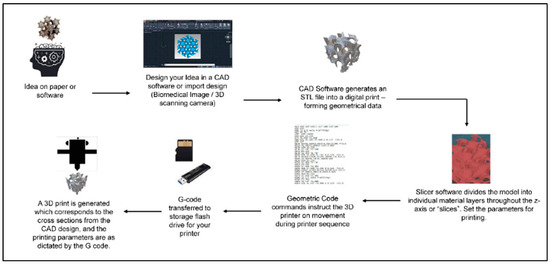
Though there are many fabrication methods when it comes to materials for biomedical applications, 3DP has resulted in significant advancements in tissue engineering. One of the benefits of 3DP is its specificity. Computer-aided design (CAD), automated slicing software (creating a stereolithography (STL) file), and typed geometric code (G-code) allow for the creation of complex, biomimetic designs that match what is seen in tissues in the human body (Figure 3). 3DP creates these designs on demand, allowing for a future in personalized medicine [31][67]. Due to the complexities of the nervous system, 3DP is an excellent method to fabricate nerve guidance conduits and hydrogels for clinical use.

Figure 3. General schematic of the computer-aided 3DP process. Figure adapted from [67]. The use of this figure is governed by the Creative Commons Attribution (CC BY license https://creativecommons.org/licenses/by/4.0/) to the licensee MDPI, Basel, Switzerland.
As discussed in previous sections, many properties contribute to biomaterials’ processability and printability. Biomaterials must be adapted for safety and efficacy in the body and withstand the fabrication, sterilization, and implantation processes. There is a similar process in the choice of the 3DP method. Although there are many types, specific techniques are more beneficial for bioprinting, and certain methods show favorable properties for printing live cells within bioinks. For example, the Advincula group has reported a number of studies using biomedically relevant polymer materials and composites for 3DP, mainly via the extrusion-based method of direct ink writing (DIW) [68][69][70][71][72]. The 3DP methods found to be best for nerve tissue engineering are photopolymerization techniques such as SLA and digital light projection (DLP), and extrusion-based techniques such as fused deposition modeling (FDM) and micro-extrusion [26][31][73].
SLA uses a UV laser to photosynthesize hydrogels or resins by immersing the stage in liquid and moving to change the layer height and resolution. Laser power, laser size, exposure time, and light wavelength can impact SLA printing. This method can be slow, costly, and dependent on specific material characteristics [29][31][73]. DLP is similar to SLA in that it uses photopolymerization. However, this method uses numerous mirrors to project the printed image, improving printing speed [29]. These photopolymerization methods are ideal for producing hydrogels for neural applications. Recently, a DLP 3D printer made photocurable silk fibroin hydrogels. Based on the data, any material with less than 10% wt./vol. hydrogel composition was difficult to print due to a loss in structural integrity. However, the hydrogel could be adjusted in a higher-range concentration (10–30%) to create physical properties, such as gelation time, swelling ratio, Young’s Modulus, porosity, and degradation time, that were optimal for nerve treatments.
FDM creates filaments using temperature changes to extrude material in a semi-liquid state, solidifying on a platform in a layer-by-layer deposition. This method is not ideal for direct cell printing if higher temperatures are required for the polymer(s). Although it is a simple, low-cost process, the prints may result in interlayer weakness depending on layer thickness, filament diameter, direction, and printing speed [29][31][73][74]. This is a standard method for printing nerve guidance conduits for PNI and SCI applications.
Micro-extrusion uses a continuous bioink stream dispensed through nozzles to fabricate structures using pistons, screws, or pneumatic pressures rapidly. Due to speed and force, this method can often exhibit too much shear stress for live cell printing, though adjustments made in these variables can exhibit favorable outcomes [31][73][74].
7. Conclusions
Regenerative medicine and nerve tissue engineering are essential research fields for developing an effective treatment for neural injuries. Numerous natural and synthetic polymers exhibit excellent biocompatible, biodegradable, and bioactive properties, in addition to excellent processability and printability. The type of polymer, method of modification, and method of fabrication can significantly impact a polymer’s physicochemical properties. These properties, such as surface chemistry, mechanical strength, and topography, greatly influence cellular adhesion, migration, proliferation, and differentiation, impacting how the neural environment affects biocompatibility and regeneration. Although numerous factors are advantageous or disadvantageous, many of these polymers, modification techniques, and fabrication methods support the safe and effective regeneration of a physical and functional nerve. They are, ultimately, moving closer toward a treatment to improve the quality of life of the millions impacted by neural injuries.
This entry is adapted from the peer-reviewed paper 10.3390/polym15183685
References
- Purves, D.; Augustine, G.; Fitzpatrick, D.; Hall, W.; LaMantia, A.; White, L.; Mooney, R.; Platt, M. Neuroscience, 6th ed.; Oxford University Press: New York, NY, USA, 2018; Available online: https://www.academia.edu/43014289/Neuroscience_by_Dale_Purves_et_al_eds_z_lib_org_ (accessed on 13 August 2023).
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131.
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365.
- Soman, S.S.; Vijayavenkataraman, S. Perspectives on 3D Bioprinting of Peripheral Nerve Conduits. Int. J. Mol. Sci. 2020, 21, 5792.
- Vella, M.A.; Crandall, M.L.; Patel, M.B. Acute Management of Traumatic Brain Injury. Surg. Clin. N. Am. 2017, 97, 1015–1030.
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795.
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9.
- Kirshblum, S.; Snider, B.; Eren, F.; Guest, J. Characterizing Natural Recovery after Traumatic Spinal Cord Injury. J. Neurotrauma 2021, 38, 1267–1284.
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Traumatic Brain Injury and Stem Cells: An Overview of Clinical Trials, the Current Treatments and Future Therapeutic Approaches. Medicina 2020, 56, 137.
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134.
- Li, Y.; Ma, Z.; Ren, Y.; Lu, D.; Li, T.; Li, W.; Wang, J.; Ma, H.; Zhao, J. Tissue Engineering Strategies for Peripheral Nerve Regeneration. Front. Neurol. 2021, 12, 768267.
- Puleo, D.A.; Bizios, R. (Eds.) Biological Interactions on Materials Surfaces; Springer: New York, NY, USA, 2009.
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for Neural Tissue Engineering. Front. Nanotechnol. 2021, 3, 643507.
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell Neurosci. 2019, 13, 33.
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonca, C.; Atayde, L.M.; Luis, A.L.; Varejao, A.S.P.; Mauricio, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918.
- Sensharma, P.; Madhumathi, G.; Jayant, R.D.; Jaiswal, A.K. Biomaterials and cells for neural tissue engineering: Current choices. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1302–1315.
- Cangellaris, O.V.; Gillette, M.U. Biomaterials for Enhancing Neuronal Repair. Front. Mater. 2018, 5, 21.
- Williams, D.F. Definitions in biomaterials. In Proceedings of the Consensus Conference of the European Society for Biomaterials, Chester, UK, 3–5 March 1986.
- Buddy, D.; Ratner, A.S.H.; Frederick, J.; Schoen, J.; Lemons, E. (Eds.) Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Inc.: Waltham, MA, USA, 2013.
- Jinku Kim, A.S.; Hollinger, J.O. An Introduction To Biomaterials; Hollinger, J.O., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2012.
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv. Healthc. Mater. 2021, 10, e2002153.
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices–regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053.
- Basics of Biocompatibility: Information Needed for Assessment by the FDA. Available online: https://www.fda.gov/medical-devices/biocompatibility-assessment-resource-center/basics-biocompatibility-information-needed-assessment-fda (accessed on 16 August 2023).
- Göktürk, E.; Erdal, H. Biomedical Applications of Polyglycolic Acid (PGA). Sak. Univ. J. Sci. 2017, 21, 1237–1244.
- Wang, Y.; Zhang, Y.; Li, X.; Zhang, Q. The progress of biomaterials in peripheral nerve repair and regeneration. J. Neurorestoratology 2020, 8, 252–269.
- Sta Agueda, J.R.H.; Chen, Q.; Maalihan, R.D.; Ren, J.; da Silva, I.G.M.; Dugos, N.P.; Caldona, E.B.; Advincula, R.C. 3D printing of biomedically relevant polymer materials and biocompatibility. MRS Commun. 2021, 11, 197–212.
- Kumar, S.S.D.; Abrahamse, H. Advancement of Nanobiomaterials to Deliver Natural Compounds for Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 6752.
- Khan, H.M.; Liao, X.; Sheikh, B.A.; Wang, Y.; Su, Z.; Guo, C.; Li, Z.; Zhou, C.; Cen, Y.; Kong, Q. Smart biomaterials and their potential applications in tissue engineering. J. Mater. Chem. B 2022, 10, 6859–6895.
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637.
- Dai, W.; Yang, Y.; Yang, Y.; Liu, W. Material advancement in tissue-engineered nerve conduit. Nanotechnol. Rev. 2021, 10, 488–503.
- Bedir, T.; Ulag, S.; Ustundag, C.B.; Gunduz, O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110741.
- Li, M.; Wang, Y.; Zhang, J.; Cao, Z.; Wang, S.; Zheng, W.; Li, Q.; Zheng, T.; Wang, X.; Xu, Q.; et al. Culture of pyramidal neural precursors, neural stem cells, and fibroblasts on various biomaterials. J. Biomater. Sci. Polym. Ed. 2018, 29, 2168–2186.
- Lin, C.; Ekblad-Nordberg, A.; Michaelsson, J.; Gotherstrom, C.; Hsu, C.C.; Ye, H.; Johansson, J.; Rising, A.; Sundstrom, E.; Akesson, E. In Vitro Study of Human Immune Responses to Hyaluronic Acid Hydrogels, Recombinant Spidroins and Human Neural Progenitor Cells of Relevance to Spinal Cord Injury Repair. Cells 2021, 10, 1713.
- Yi, S.; Xu, L.; Gu, X. Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 2019, 319, 112761.
- Xiang, W.; Cao, H.; Tao, H.; Jin, L.; Luo, Y.; Tao, F.; Jiang, T. Applications of chitosan-based biomaterials: From preparation to spinal cord injury neuroprosthetic treatment. Int. J. Biol. Macromol. 2023, 230, 123447.
- Hung, H.S.; Yang, Y.C.; Chang, C.H.; Chang, K.B.; Shen, C.C.; Tang, C.L.; Liu, S.Y.; Lee, C.H.; Yen, C.M.; Yang, M.Y. Neural Differentiation Potential of Mesenchymal Stem Cells Enhanced by Biocompatible Chitosan-Gold Nanocomposites. Cells 2022, 11, 1861.
- Amani, H.; Kazerooni, H.; Hassanpoor, H.; Akbarzadeh, A.; Pazoki-Toroudi, H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3524–3539.
- Jahromi, M.; Razavi, S.; Bakhtiari, A. The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J. Tissue Eng. Regen. Med. 2019, 13, 2077–2100.
- The Macrogalleria. Available online: https://pslc.ws/macrog/index.htm (accessed on 16 August 2023).
- Badekila, A.K.; Kini, S.; Jaiswal, A.K. Fabrication techniques of biomimetic scaffolds in three-dimensional cell culture: A review. J. Cell Physiol. 2021, 236, 741–762.
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D Printing Biocompatible Polyurethane/Poly(lactic acid)/Graphene Oxide Nanocomposites: Anisotropic Properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023.
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752.
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100.
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335.
- Onode, E.; Uemura, T.; Takamatsu, K.; Yokoi, T.; Shintani, K.; Hama, S.; Miyashima, Y.; Okada, M.; Nakamura, H. Bioabsorbable nerve conduits three-dimensionally coated with human induced pluripotent stem cell-derived neural stem/progenitor cells promote peripheral nerve regeneration in rats. Sci. Rep. 2021, 11, 4204.
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18.
- Lock, A.; Cornish, J.; Musson, D.S. The Role of In Vitro Immune Response Assessment for Biomaterials. J. Funct. Biomater. 2019, 10, 31.
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–254.
- Mariani, E.; Lisignoli, G.; Borzi, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636.
- Shen, P.; Chen, Y.; Luo, S.; Fan, Z.; Wang, J.; Chang, J.; Deng, J. Applications of biomaterials for immunosuppression in tissue repair and regeneration. Acta Biomater. 2021, 126, 31–44.
- Eppler, H.B.; Jewell, C.M. Biomaterials as Tools to Decode Immunity. Adv. Mater. 2019, 32, 1903367.
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. 3D-Printed PCL/rGO Conductive Scaffolds for Peripheral Nerve Injury Repair. Artif. Organs 2019, 43, 515–523.
- Lawkowska, K.; Pokrywczynska, M.; Koper, K.; Kluth, L.A.; Drewa, T.; Adamowicz, J. Application of Graphene in Tissue Engineering of the Nervous System. Int. J. Mol. Sci. 2021, 23, 33.
- Zhang, F.; Zhang, M.; Liu, S.; Li, C.; Ding, Z.; Wan, T.; Zhang, P. Application of Hybrid Electrically Conductive Hydrogels Promotes Peripheral Nerve Regeneration. Gels 2022, 8, 41.
- Liu, Z.; Wan, X.; Wang, Z.L.; Li, L. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications. Adv. Mater. 2021, 33, e2007429.
- Tang, M.; Song, Q.; Li, N.; Jiang, Z.; Huang, R.; Cheng, G. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials 2013, 34, 6402–6411.
- Tupone, M.G.; Panella, G.; d’Angelo, M.; Castelli, V.; Caioni, G.; Catanesi, M.; Benedetti, E.; Cimini, A. An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration. Int. J. Mol. Sci. 2021, 22, 13047.
- Kim, S.H.; Hong, H.; Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Lee, J.S.; Lee, O.J.; Lee, H.; Park, H.S.; Choi, K.Y.; et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat. Protoc. 2021, 16, 5484–5532.
- Grimaudo, M.A.; Krishnakumar, G.S.; Giusto, E.; Furlani, F.; Bassi, G.; Rossi, A.; Molinari, F.; Lista, F.; Montesi, M.; Panseri, S. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomater. 2022, 140, 88–101.
- Nelson, D.W.; Gilbert, R.J. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv. Healthc. Mater. 2021, 10, e2101329.
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505.
- Sharma, P.; Pal, V.K.; Roy, S. An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering. Biomater. Sci. 2021, 9, 3911–3938.
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782.
- Green, R.; Abidian, M.R. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv. Mater. 2015, 27, 7620–7637.
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111632.
- Polo, Y.; Luzuriaga, J.; Iturri, J.; Irastorza, I.; Toca-Herrera, J.L.; Ibarretxe, G.; Unda, F.; Sarasua, J.R.; Pineda, J.R.; Larranaga, A. Nanostructured scaffolds based on bioresorbable polymers and graphene oxide induce the aligned migration and accelerate the neuronal differentiation of neural stem cells. Nanomedicine 2021, 31, 102314.
- MacDonald, A.F.; Harley-Troxell, M.E.; Newby, S.D.; Dhar, M.S. 3D-Printing Graphene Scaffolds for Bone Tissue Engineering. Pharmaceutics 2022, 14, 1834.
- Espera, A.H., Jr.; Dizon, J.R.; Valino, A.D.; Chen, Q.; Silva, I.G.; Nguyen, D.V.; Rong, L.; Advincula, R.C. On the 3D printability of silicone-based adhesives via viscous paste extrusion. MRS Commun. 2023, 13, 102–110.
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H., Jr. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553.
- Niu, W.; Zhang, Z.; Chen, Q.; Cao, P.-F.; Advincula, R.C. Highly Recyclable, Mechanically Isotropic and Healable 3D-Printed Elastomers via Polyurea Vitrimers. ACS Mater. Lett. 2021, 3, 1095–1103.
- Siacor, F.D.C.; Chen, Q.; Zhao, J.Y.; Han, L.; Valino, A.D.; Taboada, E.B.; Caldona, E.B.; Advincula, R.C. On the additive manufacturing (3D printing) of viscoelastic materials and flow behavior: From composites to food manufacturing. Addit. Manuf. 2021, 45, 102043.
- Chen, Q.; Zhao, J.; Ren, J.; Rong, L.; Cao, P.F.; Advincula, R.C. 3D Printed Multifunctional, Hyperelastic Silicone Rubber Foam. Adv. Funct. Mater. 2019, 29, 1900469.
- Yu, K.; Zhang, X.; Sun, Y.; Gao, Q.; Fu, J.; Cai, X.; He, Y. Printability during projection-based 3D bioprinting. Bioact. Mater. 2022, 11, 254–267.
- Abelseth, E.; Abelseth, L.; De la Vega, L.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater. Sci. Eng. 2019, 5, 234–243.
This entry is offline, you can click here to edit this entry!
