Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Adenotonsillar hypertrophy has been well-acknowledged as the primary instigator of sleep-disordered breathing in the pediatric population. This condition spans a spectrum, from typical age-related growth that the immune system influences to persistent pathological hypertrophy. Reduction in air spaces, metabolic changes, neurobehavioral alterations, and chronic inflammation characterizes the latter form. As the go-to treatment, adenotonsillectomy has proven effective.
- craniofacial development
- craniofacial anomaly
- obstructive sleep apnea
- sleep-disordered breathing
1. Introduction
The development of skeletal and facial alterations is undoubtedly influenced by an amalgamation of diverse factors, ranging from genetics and diet to oral behavior and human development [1]. These intrinsic and extrinsic factors can come into play during fetal development and persist throughout an individual’s life, ultimately affecting the likelihood of developing OSA and related SDB conditions.
Exploring Theories on Craniofacial Structure and SDB:
In the quest to understand the relationship between craniofacial structure and SDB, two prominent theories have emerged the “gracilization theory” [2] and the “gravitational hypothesis” [3]. These theories offer distinct physiological mechanisms that underpin the association between craniofacial structure and SDB.
The Gracilization Theory:
The gracilization theory revolves around evolutionary diet and facial anatomy changes over time. It suggests that alterations in diet and lifestyle have contributed to the gradual refinement and “gracilization” of the facial structure. Such changes may influence the airway dimensions and respiratory mechanics, potentially leading to an increased susceptibility to SDB.
The Gravitational Hypothesis:
On the other hand, the gravitational hypothesis highlights the significance of body position during sleep and the role of gravity in affecting the upper airways. When individuals assume specific sleeping postures, the influence of gravity on the upper airway can potentially lead to airway collapse and obstruction, contributing to SDB. This theory postulates that the body’s positioning during sleep interacts with the anatomical features of the upper airway, shaping the development of SDB [4].
2. Gracilization Hypothesis
The gracilization theory, put forth by Weber in 2023 [5], posits that changes in the human diet over time, as supported by Luca et al. in 2010 [6], have weakened the masticatory muscles. Consequently, this has influenced the craniofacial structure, reducing the volume of the upper airways. According to this theory, the mandible and tongue have evolved to be less voluminous to adapt to dietary shifts, including consuming solid foods and developing language. However, this reduction in tongue space within the oral cavity may contribute to airway obstruction during sleep, as discussed by Katz et al. in 2017 [7].
Additionally, the gracilization hypothesis proposes that the decrease in the robustness of the human skeleton and facial bones can be attributed to the transition from an active foraging lifestyle and a diet of raw foods to a more sedentary lifestyle and an agricultural diet. Lacruz et al. [8] support this notion, stating that this transition has reduced bone mass and decreased mechanical load on the jaws during chewing.
Recognizing that these theories offer possible explanations for the connection between craniofacial changes and SDB is essential (Figure 1). However, further research is required to fully comprehend the intricate interplay between diet, craniofacial conformation, and the development of airway obstruction during sleep [1][9].
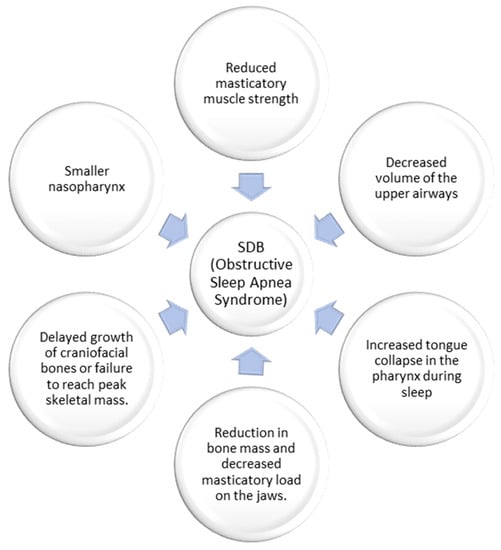
Figure 1. The hypothesis of gracilization on the genesis of sleep-disordered breathing.
Eimar et al. conducted two cross-sectional studies to explore the impact of OSA on bone density, using Mandibular Cortical Width (MCW) as a measure. The studies revealed that children with OSA had lower MCW than controls, suggesting a potential link between OSA and bone density. The authors proposed that an inability to attain maximum skeletal mass or delayed growth of craniofacial bones might lead to a reduced nasopharynx size, potentially playing a role in the emergence of SDB [10]. Similarly, a study by Fernandes Fagundes et al. [11] observed reductions in MCW among children with OSA or those at high risk of OSA, indicating potential interactions between mandibular bone development and pediatric OSA.
Skeletal alterations and oral breathing can result in changes in the position of the maxillary palate and mandibular body relative to the mandibular ramus, leading to an increased gonial angle [12]. These changes can affect various structures, including the maxillary alveolar crest, the vomer, and the ethmoid, leading to septal deformities and retroclination of the pre-maxilla and its incisors. The development of OSA has been associated with a reduction in the size of the hard palate, which can subsequently decrease the available space for the tongue [13][14].
During sleep, the reduced retaining mechanism within the oral cavity can cause the tongue to collapse into the pharynx, increasing the likelihood of OSA worsening. This condition can further impede airflow and exacerbate symptoms of SDB [15].
In conclusion, the gracilization theory posits that changes in the human diet have reduced masticatory muscle strength, shaped the craniofacial region, and result in a decreased upper airway volume. This reduction in airway space increases the risk of tongue collapse during sleep, contributing to OSA. Additionally, some researchers propose that gracilization is linked to transitioning from a raw food-based diet to a sedentary lifestyle and agricultural diet, leading to reduced bone mass and jawbone load.
Furthermore, studies suggest that children with OSA exhibit lower bone density than those without the condition. This lower bone density could be attributed to the failure to achieve optimal skeletal mass or delayed craniofacial bone growth, leading to a smaller nasopharynx and consequent SDB.
These findings provide valuable insights into the intricate relationship between craniofacial anatomy, the human diet, and the development of OSA. However, further research is essential to comprehensively understand these factors’ complex interactions and develop practical preventive and therapeutic approaches for OSA.
3. The Impact of Gravity on Sleep-Disordered Breathing: Unraveling the Complexity
Sleep, a fundamental aspect of human existence, is a physiological state during which the body undergoes various intricate processes. Among them, the loss of muscle tone during sleep can lead to the collapse of the upper airways, giving rise to conditions such as apnea and snoring [16]. However, the influence of gravity on sleep-related breathing disorders (SDB) is a captivating facet that warrants exploration.
Gravity exerts its force on the body, mainly when one adopts a supine position (lying on the back) during sleep. This positioning can trigger increased compression and narrowing of the upper airways, accentuating the likelihood of SDB [17]. Remarkably, the impact of gravity extends beyond mere airway mechanics, extending its grasp to influence craniofacial conformation, consequently contributing to SDB (Figure 2).
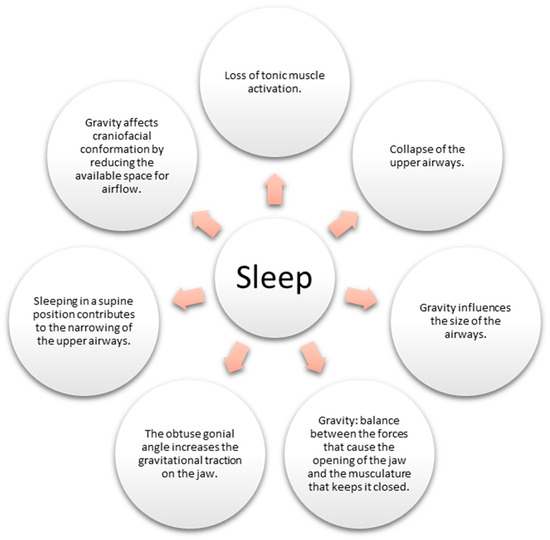
Figure 2. Impact of gravity on the airways during sleep.
Gravity also orchestrates the ballet of oral breathing, regulating the interplay of forces determining jaw movement. The delicate balance between the forces promoting jaw opening and those ensuring its closure is influenced by this gravitational pull [18]. Studies have suggested that an obtuse gonial angle could favor oral breathing by increasing the gravitational force on the jaw [18].
Intriguingly, investigations conducted on astronauts in gravity-deficient environments have unveiled a positive influence on sleep-related breathing. The absence of gravity in such settings correlates with reduced apnea and snoring incidents [19].
To comprehend the pathophysiology of SDB disorders comprehensively, it is imperative to acknowledge the combined effect of muscle tone loss and gravitational influence on the collapse and constriction of the upper airways [16]. Moreover, gravity’s impact on the human body is far from uniform, varying considerably based on individual body position and anatomical structure. Thus, a comprehensive exploration of gravity’s influence on the airways and sleep necessitates further research and investigation [17].
In conclusion, the intricate relationship between gravity and SDBs is a captivating study area that illuminates human physiology’s complexities during slumber. Understanding the interplay between muscle tone loss, gravity’s influence on the upper airways, and its impact on oral breathing is crucial in comprehending the pathophysiology of SDB.
This entry is adapted from the peer-reviewed paper 10.3390/children10081426
References
- Ryan, T.M.; Shaw, C.N. Gracility of the modern Homo sapiens skeleton is the result of decreased biomechanical loading. Proc. Natl. Acad. Sci. USA 2015, 112, 372–377.
- Ledogar, J.A.; Dechow, P.C.; Wang, Q.; Gharpure, P.H.; Gordon, A.D.; Baab, K.L.; Smith, A.L.; Weber, G.W.; Grosse, I.R.; Ross, C.F.; et al. Human feeding biomechanics: Performance, variation, and functional constraints. PeerJ 2016, 4, e2242.
- Marques, M.; Genta, P.R.; Sands, S.A.; Azarbazin, A.; de Melo, C.; Taranto-Montemurro, L.; White, D.P.; Wellman, A. Effect of Sleeping Position on Upper Airway Patency in Obstructive Sleep Apnea Is Determined by the Pharyngeal Structure Causing Collapse. Sleep 2017, 40, zsx005.
- Saint-Fleur, A.L.; Christophides, A.; Gummalla, P.; Kier, C. Much Ado about Sleep: Current Concepts on Mechanisms and Predisposition to Pediatric Obstructive Sleep Apnea. Children 2021, 8, 1032.
- Weber, G.W. Quantum Leaps in Human Biocultural Evolution and the Relationship to Cranial Capacity. Life 2023, 13, 1030.
- Luca, F.; Perry, G.H.; Di Rienzo, A. Evolutionary adaptations to dietary changes. Annu. Rev. Nutr. 2010, 30, 291–314.
- Katz, D.C.; Grote, M.N.; Weaver, T.D. Changes in human skull morphology across the agricultural transition are consistent with softer diets in preindustrial farming groups. Proc. Natl. Acad. Sci. USA 2017, 114, 9050–9055.
- Lacruz, R.S.; Stringer, C.B.; Kimbel, W.H.; Wood, B.; Harvati, K.; O’Higgins, P.; Bromage, T.G.; Arsuaga, J.L. The evolutionary history of the human face. Nat. Ecol. Evol. 2019, 3, 726–736.
- Eyquem, A.P.; Kuzminsky, S.C.; Aguilera, J.; Astudillo, W.; Toro-Ibacache, V. Normal and altered masticatory load impact on the range of craniofacial shape variation: An analysis of pre-Hispanic and modern populations of the American Southern Cone. PLoS ONE 2019, 14, e0225369.
- Eimar, H.; Al-Saleh, M.A.Q.; Cortes, A.R.G.; Gozal, D.; Graf, D.; Flores-Mir, C. Sleep-Disordered Breathing Is Associated with Reduced Mandibular Cortical Width in Children. JDR Clin. Trans. Res. 2019, 4, 58–67.
- Fernandes Fagundes, N.C.; d’Apuzzo, F.; Perillo, L.; Puigdollers, A.; Gozal, D.; Graf, D.; Heo, G.; Flores-Mir, C. Potential impact of pediatric obstructive sleep apnea on mandibular cortical width dimensions. J. Clin. Sleep Med. 2021, 17, 1627–1634.
- Hyman, A.J.; Fastenberg, J.H.; Stupak, H.D. Orientation of the premaxilla in the origin of septal deviation. Eur. Arch. Otorhinolaryngol. 2019, 276, 3147–3151.
- Kim, J.H.; Guilleminault, C. The nasomaxillary complex, the mandible, and sleep-disordered breathing. Sleep Breath. 2011, 15, 185–193.
- D’Ascanio, L.; Lancione, C.; Pompa, G.; Rebuffini, E.; Mansi, N.; Manzini, M. Craniofacial growth in children with nasal septum deviation: A cephalometric comparative study. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1180–1183.
- Ito, E.; Tsuiki, S.; Maeda, K.; Okajima, I.; Inoue, Y. Oropharyngeal Crowding Closely Relates to Aggravation of OSA. Chest 2016, 150, 346–352.
- Ayappa, I.; Rapoport, D.M. The upper airway in sleep: Physiology of the pharynx. Sleep Med. Rev. 2003, 7, 9–33.
- Huang, Y.S.; Guilleminault, C. Pediatric Obstructive Sleep Apnea: Where Do We Stand? Adv. Otorhinolaryngol. 2017, 80, 136–144.
- Crupi, P.; Portelli, M.; Matarese, G.; Nucera, R.; Militi, A.; Mazza, M.; Cordasco, G. Correlations between cephalic posture and facial type in patients suffering from breathing obstructive syndrome. Eur. J. Paediatr. Dent. 2007, 8, 77–82.
- Elliott, A.R.; Shea, S.A.; Dijk, D.J.; Wyatt, J.K.; Riel, E.; Neri, D.F.; Czeisler, C.A.; West, J.B.; Prisk, G.K. Microgravity reduces sleep-disordered breathing in humans. Am. J. Respir. Crit. Care Med. 2001, 164, 478–485.
This entry is offline, you can click here to edit this entry!
