Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
The rampant spread of the COVID-19 infection poses a grave and formidable challenge to global healthcare, with particular concern to the inhabitants of the African continent. In response to these pressing concerns, different strategies have been employed to combat the emergence of this insidious disease, encompassing crucial measures such as physical distancing, the utilization of face masks, meticulous hand hygiene, and widespread vaccination campaigns.
- transfer
- COVID-19
- technology
- vaccine
1. Introduction
The COVID-19 pandemic, an all-encompassing global threat, has left no region untouched in the entire world [1]. As of 9 July 2023, the tally of confirmed COVID-19 cases stands at a staggering of 767,972,961, with a heart-wrenching death toll of approximately 6,950,655 worldwide [2]. Data profiles from the World Health Organization (WHO) in data records, as shown in Table 1, further shows the recorded cases across the population, as alarming reports continue to pour in regarding the surge of COVID-19 cases in low- and middle-income countries (LMICs) like Bangladesh, Cameroon, the Democratic Republic of Congo, India, Indonesia, Nigeria, Mali, Gambia, Senegal, Ghana, Sudan, Tunisia, and various other African nations [3,4]. Africa, in particular, has confirmed cases of 9,542,363 and the death toll reaching 175,399 as of 9 July 2023. Tragically, the scarcity or absence of COVID-19 vaccines in many nations has translated into an escalating number of lives lost. In the initial stages of the pandemic, when neither a COVID-19 vaccine nor treatment options were known, the concept of herd immunity emerged as a potential remedy for combating the SARS-CoV-2 virus [1].
Table 1. COVID-19 cases in each continent and the respective population size as of 16 August 2023.
| WHO Regions | Total Cases (Million) | Population (Billion) |
|---|---|---|
| Europe | 249.09 | 0.74481 |
| Asia | 299.92 | 4.72 |
| North America | 124.41 | 0.60032 |
| South America | 68.8 | 0.43882 |
| Africa | 13.11 | 1.43 |
| Oceania | 14.43 | 0.04504 |
Amidst this relentless surge, multiple nations have made significant strides in developing vaccines against the disease. Notably, certain high-income countries (HICs) have made remarkable progress in the manufacturing and development of COVID-19 vaccines [5]. Concurrently, numerous vaccine candidates have received emergency use authorizations, leading some countries to commence extensive vaccination campaigns [5]. However, the mere existence of licensed vaccines falls short of achieving global control over COVID-19. It is imperative to ramp up production on a larger scale, ensuring affordability, broad coverage, and equitable distribution across the global population. This encompasses addressing the evolving genetic makeup of the virus, particularly in Africa, where unique host—virus interactions have given rise to new variants [6]. Consequently, the World Health Organization (WHO) and its collaborative partners have diligently worked towards enhancing worldwide access to COVID-19 vaccines. Their efforts involve bolstering the capabilities of low- and middle-income countries (LMICs) to facilitate vaccine production, thereby curbing the pandemic’s impact [7].
To facilitate the manufacturing process of LMICs, the WHO is launching a project in South Africa, providing technical expertise for vaccine production, empowering the continent to combat the epidemic, and attain self-sufficiency [8]. The success of vaccine technology transfer to Africa hinges upon the region’s preparedness to embrace this knowledge hub, with far-reaching implications for the vaccine’s efficacy. The implementation of effective strategies is essential to overcome barriers hindering the seamless transmission of expertise.
2. Rationale for COVID-19 Vaccine Technology Transfer in Africa
On 11 March 2020, the World Health Organization (WHO) officially declared the COVID-19 pandemic, marking a turning point in global health [9]. Notably, as of 18 November 2022, Africa accounted for 3.48% of the total worldwide infections, with 12,693,549 confirmed cases. South Africa bore the brunt of the outbreak on the African continent, reporting approximately 4,036,623 infections, making it the most heavily impacted country on the continent [10]. It is important to note that Africa’s lower number of confirmed cases and death rates compared to other continents may be attributed to limited COVID-19 testing, which skews the incidence rate and fails to provide an accurate depiction of the outbreak’s magnitude [9].
Infectious diseases remain a leading cause of death in Africa and Asia, particularly among children under the age of five. In 2016, Sub-Saharan African countries accounted for 44.4% of all child fatalities, while South Asian countries accounted for 248%. Shockingly, children in low- and middle-income nations face a mortality risk 34 times higher than their counterparts in high-income countries [11]. This underscores the urgent need for targeted interventions and healthcare improvements to safeguard the well-being of vulnerable populations in these regions.
At the end of June 2023, an estimation of 13 billion vaccine doses had been administered worldwide, with a meagre 0.2% allocated to low-income countries, especially in Africa and the Oceania continent, as depicted in Figure 1 below [12]. WHO statistics, as reported by Id and colleagues [3] in 2020, revealed the ongoing spread of COVID-19 in densely populated regions of Ghana, Nigeria, the Democratic Republic of Congo, and potentially across the African continent. This emphasises the imperative of a global commitment to developing a safe and affordable COVID-19 vaccine, with a particular emphasis on prioritizing vaccination efforts in African nations. Dr. Seth Berkley, the CEO of GAVI (the Vaccine Alliance), has underscored the crucial importance of addressing the specific vaccine needs of these African countries, making it an urgent global priority.
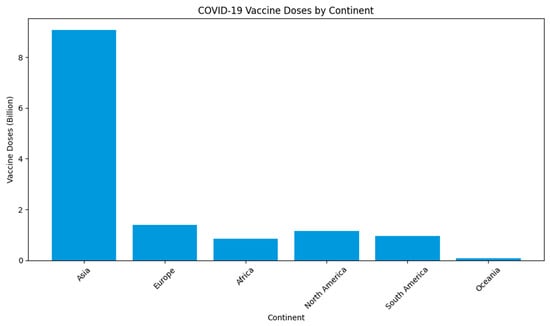
Figure 1. Total COVID-19 vaccine doses (billion) across the continent.
In 2021, the World Health Organization (WHO) established a consortium in South Africa to establish the inaugural COVID mRNA vaccine technology transfer hub [13]. Vaccine production entails various challenges, such as process development, maintenance, lead time, manufacturing facilities, equipment, life cycle management, and product portfolio management [11]. The WHO highlights the critical importance of a robust and stable production process, as well as consistent component supply over an extended period, ensuring the vaccine’s longevity in the market. When considering investments in vaccine production in Africa, a careful evaluation of these factors is essential. Neglecting to address these risks may result in costly product recalls, market suspensions, and penalties if a manufacturer fails to fulfil supply agreements.
In a study by Asundi in 2020 [14], the advancement of next-generation vaccines based on mRNA, viral vector, or protein subunit technologies presents promising opportunities to alleviate the impact of infectious diseases. The utilisation of these technologies during the COVID-19 pandemic demonstrated the speed at which new vaccines can be developed. However, challenges arise when it comes to scaling up vaccine production to achieve global vaccine equity, highlighting the obstacles that these innovative technologies still face in meeting global demand. In medical technology development, legal barriers such as intellectual property transfer are widely recognized.
In collaborative efforts with Biovac, Afrigen Biologics and Vaccines, a network of universities, and the Africa Centers for Disease Control and Prevention (ACDC), the WHO and its COVAX partners are actively working towards establishing the first COVID-19 mRNA vaccine technology transfer hub in South Africa [13].
The establishment of vaccine production capacity, including for COVID-19, presents unique challenges in Africa. Many African countries encounter obstacles such as limited knowledge, scarcity of raw materials, consumables, and equipment, market access restrictions, import policies, regulatory limitations in good manufacturing practice (GMP) inspection, and lengthy delays in dossier review and clearance [11]. Additionally, essential factors such as facility development, financial support, and technology acquisition must be considered. These challenges are compounded by other prevalent issues in Africa, such as inadequate infrastructure, a shortage of healthcare professionals, diverse religious landscapes, and uneven population distribution [15].
3. The Ongoing Endeavours in Africa to Develop Domestically Manufactured and Approved COVID-19 Vaccines
More than 80% of Africa’s population remains unvaccinated, largely due to the unequal availability of vaccine production, of which the available ones are concentrated in a few high-income nations in Africa [16]. The COVID-19 pandemic shed light on Africa’s heavy reliance on imported immunizations, with 99% of vaccines being sourced externally from other parts of the world [17]. Consequently, African governments have faced challenges in vaccinating their populations. The news of the German biotechnology company BioNTech establishing COVID-19 vaccine manufacturing facilities in Rwanda and Senegal brought hope to many African countries [18].
Inadequate and unreliable vaccine supply, coupled with unequal distribution, poor delivery networks, and infrastructure limitations, have hindered mass vaccination efforts across the continent. Transferring vaccine technology to African countries is seen as a positive step toward enhancing immunisation coverage throughout Africa [18]. Some African countries are actively working on developing their own approved vaccines, aiming to improve self-sufficiency [19]. Currently, only 10 African vaccine manufacturers are situated in five countries: Egypt, Morocco, Senegal, South Africa, and Tunisia. However, the majority of local businesses in Africa focus on packaging, labelling, and occasionally filling and finishing, with limited upstream manufacturing capabilities [20].
To address these challenges, the US International Development Finance Corporation, in collaboration with European partners, announced a EUR 600 million (USD 710 million) financing package for South Africa’s Aspen Pharmacare. By the end of 2023, Aspen’s factory is expected to produce millions of doses and engage in the “fill-and-finish” process for approximately 500 million Johnson & Johnson doses [21]. Additionally, South Africa’s Biovac Institute has taken the responsibility of accelerating the Pfizer vaccine fill-and-finish production in Cape Town, starting in 2022. In Senegal, the government is partnering with the Foundation Institute Pasteur de Dakar, with support from the US and Europe, to establish a USD 200 million COVID-19 vaccine manufacturing facility that will encompass both the production of vaccine substances and fill-and-finish operations [21]. Moreover, Egypt has plans to manufacture Chinese Sinovac at a new Vacsera facility near Cairo, with a capacity of 1 billion vaccines per year. Egypt is also exploring agreements for drug substance manufacture and fill-and-finish of Russia’s Sputnik V vaccine, aiming to reduce Africa’s reliance on vaccine imports [21].
The Coalition for Epidemic Preparedness Innovations (CEPI) and the Institute Pasteur de Dakar (IPD) have formed a partnership to promote equitable access to vaccines in Africa and support the African Union’s goal of increasing the share of vaccine supply from African manufacturers to 60% by 2040. This collaboration aims not only to establish COVID-19 vaccines but also advance vaccine development for other diseases [22].
This entry is adapted from the peer-reviewed paper 10.3390/life13091886
This entry is offline, you can click here to edit this entry!
