Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Human microbiome has not been at the center of scientific research until recent years, when the scientific approach to the gut–brain axis and its medical involvement in multiple pathologies has revealed the decisive role of the intestinal flora.
- functional
- microbiome
- gut–brain axis
- probiotics
1. Introduction
Human microbiome has not been at the center of scientific research until recent years, when the scientific approach to the gut–brain axis and its medical involvement in multiple pathologies has revealed the decisive role of the intestinal flora.
The most important implications for the microbiome, and the gut–brain connection, reside within the Functional Gastrointestinal Disorders (FGID) which have been recently linked to psychiatric symptoms. Interestingly, it seems that for the majority of patients, no matter the primary complain, whether it is psychiatric or gastrointestinal, the other end of the axis will clinically manifest at some point. This is the main reason why these cases often had poor therapeutical response until recently [1].
2. Review of Gut–Brain Axis and the Microbiome Physiology
A perfect and balanced coordination between the central nervous system and the enteric environment requires a complex and delicate calibration involving sympathetic and parasympathetic relays, hormonal and neurotransmitter activation and the autonomous nervous function. Through these pathways, there is a bidirectional regulation and influence over cognitive, emotion, behavior on one side and the functionality of the gastrointestinal system, with all roles involved: digestion, absorption and enteric immune system [2,3]. The proportion and influence level that the two systems exert on each other are still subjects of debate but it is clear that they influence each other by efferent and afferent pathways and the physiologic balance is affected heavily by outer factors, both from outside and inside the body. Beyond the direct relationship between central nervous system and gastrointestinal system, there are outer elements that influence the axis in negative or positive proportions, such as nutrients, pharmacological treatments, social and environmental factors, stress, behavior, mood and genetics [4].
2.1. The Microbiome
Most of the present literature supports the theory that describes the gastrointestinal microbiome and its specific strains as a main modulator of the gut–brain axis, acting decisively in functional gastrointestinal pathophysiology with manifesting psychiatric features. The hypothesis begins from simple clinical observations of antibiotic-treated patients with hepatic encephalopathy and afterwards, studies on germ-free animals have further demonstrated the importance of the microbiome in the development and physiology of the central nervous system. Nowadays, there is no doubt about the major implications of the microbiome in the homeostasis of the gut–brain axis and its involvement in many more medical fields, especially psychiatry and neurology. Furthermore, it seems that every species of bacteria has a specific function such as maintaining the intestinal barrier integrity [5], modulating afferent sensory nerves’ calcium-dependent potassium channels [6], promoting local neurotransmitter precursors for GABA, serotonin, acetylcholine [7], stimulating the sympathetic nervous system through bacterial metabolites such as butyric acid, propionic acid or acetic acid [8] and lastly, mediating mucosal immune activity through the enteral nervous system, P substance and proteases [9]. Additionally, the mechanism through which the microbiome influences the gut–brain axis mechanism is related to the vague nerve [10]. The present article, however, focuses on the microbiome and the gut–brain axis functions related to functional digestive disorders and coexisting psychiatric symptoms.
As such, modulating the composition of the gut microbiome and identifying its specific strain disbalance may be a future target in the management of functional gastrointestinal diseases and neuro–psychiatric-associated pathology. [11].
Given the current knowledge, the best definition of the interactions between the GI tract and the brain is the microbiome–gut–brain axis, as a recognition for the important role of the microbiome. Although the interaction per se is a synergic mechanism that modulates multiple physiological variables, it is important to understand the influence of every direction, as most new hypotheses state that every pathway of the axis is a key for unlocking therapeutic targets in different diseases [12].
2.2. Brain to Gut Connection and Gut to Brain Connection
The interaction between the central nervous system and the intestinal environment is based on neuronal, hormonal, immune and metabolic reactions. On one hand, the central nervous system is capable of influencing the local enteral nervous system, activating the hypothalamic pituitary adrenal axis and signaling through the vague nerve which will alter enteral environment through mucus production, permeability of the intestinal wall and immune reactivity that will further alter the microbiome strain population, causing more enteral disbalance that will redirect pathologic signals back to the brain. As such, the specific separate role of each efferent and afferent pathway and their synergy function with the microbiome is vital for understanding its pathophysiology [13].
The individuality of the two pathways and the proportions of each function inside the axis were described in a study in 2016 led by Keightley, that observed patients with functional digestive disorders and psychiatric symptoms. The study separated the patients into two groups, by baseline complaints and symptoms and the clinical aspects that appeared at 1 year follow-up. The group with digestive primary complaints had significantly more anxiety and depressive symptoms at 1 year follow-up and the majority of the patients with anxious and depressive baseline complaints had developed irritable bowel syndrome or other functional digestive symptoms at 1 year follow-up. Additionally, the most important changes were in the digestive baseline symptoms’ group, which suggest higher afferent influence than on the efferent pathway. These findings suggest that the gut–brain axis can be analyzed individually, by starting from each end, but also, that the gut and microbiome could exert more influence on the brain than initially thought [14].
2.3. Functional Mechanisms and Pathways between Microbiome, Gut and Brain
The three centers of the axis work individually as well as in a synergic cooperation keeping the sensitive balance with the help of nervous, immune and endocrine systems. Each physiologic pathway is a possible therapeutic target and should be assessed as a specific as well as integrated mandatory part of the whole mechanism [15].
2.3.1. The Autonomous Nervous System
The autonomous nervous system maintains the homeostasis of the GI tract by managing endocrine, motor and behavioral signals by linking both brain and digestive systems. The autonomous nervous system controls the information received from the central nervous system and neuro–endocrine axis and assures intestinal responses in the form of permeability, motility, mucosal state and enteral immune responses, this being the efferent way. On the afferent path, distress, dysbiosis, and pain local signals are modulated and transmitted to the central nervous system via sympathetic and parasympathetic system, triggering responses in the connected cerebral areas [16]. Furthermore, it has been demonstrated that the microbiome is capable of interaction with the autonomous nervous system through microbial metabolites that act similar to excitatory or inhibitory triggers on the sympathetic and parasympathetic network. Tryptophan, catecholamines and serotonin have direct influence over the afferent pathways, inducing mood or cognitive changes.
The main communication path is apparently the vagus nerve, acting similar to a two-way highway, that is supposed to transport signals directly from microbiome and local alteration stimuli but also directing responses from the brain. A study in 2005 was relevant in this direction as it demonstrated the direct activation of vagal ganglia and relay nucleus in the medulla oblongata after Campylobacter gut inoculation in mice, followed by anxiety-like behavior. The study was relevant for future therapeutic modulation of the gut–brain axis through its fastest route that is the vagus nerve [17]. Vagal afferents transmit information from the gut with decreasing fiber density from the duodenum to the transverse colon with terminal connectivity in the lamina, soft muscle, mucosa and in some of the neuroendocrine cells. The gut level synapses are capable of detecting all alterations in the intestinal environment, each afferent having specific roles and, translating specific responses from the nervous system [18]. Modulating inflammatory responses and mood/affective responses to local gut dysfunction are also demonstrated to be managed by the vagus. The most relevant observations in this direction were the studies on vagotomized animals and humans. Vagotomy, as part of peptic ulcer treatment was reported to raise the incidence of psychiatric disorders in the studied population versus control In addition, probiotics have been studied recently as adjuvant therapy in anxiety and depression with promising results but it has been observed that positive results due to administering Lactobacillus rhamnosus are not the same in vagotomized animals compared to controls [19].
2.3.2. The Enteric Nervous System
One of the most important relays in the gut–brain connection consists of the enteral nervous system. It is capable of acting independently or as a part of the autonomous nervous system. As part of the brain–gut axis, it serves as a transmitter of information to the brain via vague nerve afferents and given the majority of afferent fibers, it is presumed that this pathway of the axis acts more like a transmitter. Moreover, it is known that the enteric nervous system can function independently if the vague connection to the brain is severed, continuing to manage local bowel mechanisms and homeostasis [20]. In a physiologic environment, the local bowel disruptions will signal the brain and will turn on the vago–vagal reflexes which will regulate motility, mucosal functions and even microbiome balance. The enteric nervous system also transmits satiety or nausea sensations to the central nervous system which, apparently, bypasses consciousness but still, alters mood behavior and cognition. Given the above, the modulation of the gut–brain axis should approach the enteric nervous system as much as an independent mechanism and as an important relay of the gut–brain axis. The enteric nervous system is currently under study as it may be involved in neuro-degenerative disorders but also in spectrum disorders as autism has the most GI comorbidities amongst primary psychiatric diseases [21].
2.3.3. The Enteric Immune System
The interface between microbiome and intestinal tissue consists of a dense mucosal layer that acts as a protectant but also as a center for coordination between internal environment and the intestinal lumen. At this level, there is a mandatory immune mechanism to manage that interface and maintain a synergic collaboration between intra-luminal and extra-luminal systems. As such, the enteric immune system must recognize self-antigens and eliminate potential harmful microorganisms. Moreover, the intestinal epithelium contains various cells that can trigger immune responses and release pro-inflammatory substances. [22] The immune modulation of the gut–brain axis resides in the microglial activity. First of all, connections between microglia and the microbiome are scarcely studied but there seems to be a strong belief that the microbiome is modulating the microglial development and activity [23]. Furthermore, the strong connection between microglia and microbiome was demonstrated in an animal model study, in which germ-free mice were exposed to a neurotropic virus. The germ-free mice were unable to stimulate anti-viral immunity and the lack of microglial activity led to demyelination. The physio–pathologic process was still reversible as the mice’s intestinal environment was repopulated by healthy microbiome [24].
Microbiome modulated microglial activity in the gut–brain axis consists of triggering immune responses, on the one hand, through a general immune response and especially, monocytes and further through TNF-α, interleukins and immunoglobulins and, on the other hand, through specific immune responses through lymphoid cells [25,26]. Studies highlight the role of microbiome in managing neuroimmune responses. As such, it seems that CD4 and CD8 lymphocytes were reprogrammed into T-cells and used as immunoregulators following L. reuteri treatment that generated tryptophan derivates and activated specific receptors in CD4. Of course, the research is in animal model stages but results are relevant and bring new perspectives upon neuro-immunity and microbiome importance within the gut–brain axis [27]. Even more so, lymphocyte deficiency resulted in cognitive disruptions and anxiety symptoms that were treated with lactobacillus species probiotics. This is yet another statement in favor of the synergy between neuro-immunity mechanisms inside the gut–brain axis and the microbiome [28].
2.3.4. The Neuroendocrine System
The neuroendocrine enteric system seems to be connected to the enteric nervous system and to the microbiome, being currently under research for its possible therapeutic implications in metabolic diseases, especially in the type II diabetes. Neuroendocrinal representatives are the entero-chromaffin cells and the enteroendocrine L cells. Both types of cells are dispersed throughout the distal small intestine and colon, as well as the majority of the flora resides but also, they make contact with most of luminal constituents and as well, with enteric nervous and immune systems. The interactions with the microbiome are still under study and not yet clear but it is certain that microbial metabolites can trigger neuroendocrine responses through its specific cells [29].
Enterochromaffin cells were demonstrated in animal studies to interact and respond to microbial metabolites in the colonic lumen by expressing and activating 5-hydroxytryptamine (5-HT), from tryptophan and signal afferent vagal pathways, modulating peristatic function, pain or inflammation [30]. Moreover, another study revealed that increasing the population of Clostridium could elevate the 5-HT expression and accelerate intestinal transit. The ideas of this study are currently investigated for possible Crohn’s disease future research [31]. The enterochromaffin cells’ activity is not likely to impact the brain directly but it could interact with the gut–brain axis via vagal fibers as 5-HT does not cross the blood–brain barrier. The indirect impact of 5-HT has been demonstrated in chemotherapy as nausea and vomiting are produced by massive discharge of 5-HT and altered signaling in the gut [32]. Irritable bowel syndrome seems to undergo the same altered 5-HT expression as the new hypotheses state [33].
Enteroendocrine L cells are responsible for the expression of YY peptide (PYY) and glucagon-like peptide (GLP-1). The receptors for these are widely expressed throughout the gut and central nervous system, involving even the hypothalamic axis. The role of this system is transmitting information about food intake and satiety to the brain. Given the potent connection to the central nervous system, the peptides secreted by enteroendocrine L cells are known to have major implications in eating disorders, especially anorexia [34]. If in the proximal area of the gut, the enterochromaffin cells respond to nutrients and activate peptide release, in the distal it seems there are L cells that are almost entirely engaged in bacterial metabolite interactions [35]. The role of the neuroendocrine peptides in eating disorders and metabolic diseases and its interactions with the microbiome is gaining interest in the research field. As studies have stated, prebiotic (polysaccharides) and probiotic (Lactobacillus) supplementation results in increasing levels of GLP-1 and PPY with further reduction of food intake and insulin resistance [36,37].
2.4. The Hypothalamic–Pituitary–Adrenal Axis (HPA Axis)
The HPA axis is a key player within the gut–brain axis as it reacts to both brain and gut signals and assures a bidirectional response to stress. Studies have revealed an important role of the HPA axis in the gut environment of pediatric patients, a well as infants and children [38]. In animal models and human studies, prebiotic therapy altered the HPA-axis response, probably by indirect manipulation of gut microbiome [39].
2.5. Spinal Cord within the Gut–Brain Axis
The spinal cord acts like a signal carrier for pain and distress to the brain, via spinothalamic, spinomesencephalic and spinoreticular tracts. The routes conduct location and intensity and nociceptive signals that will be processed in emotional and behavioral areas of the brain and furthermore, respond with excitatory or inhibitory signals [40]. Following brain injury models, studies have demonstrated a bidirectional coordination between spinal cord and gut microbiome with mutual altering responses at distress stimulation; more specifically, spinal cord lesions promote microbiome imbalances that will maintain a negative feedback on the spinal cord [41].
2.6. The Neurotransmitters
Neurotransmitters have been studied for a long time as they are supposed to be the main linkage between functional digestive symptoms and neuropsychiatric disorders, affecting the digestive mechanism and mood, behavior and cognition at the other end.
Within the gut–brain axis, there is a strong crosstalk with the microbiome as gut bacteria are capable of producing β-glucuronidase that activates dopamine and epinephrine. In addition, catecholamines can be directly produced by some species of bacteria such as Bacillus and by doing so, enhance the neuroendocrine communication between gut and brain [42].
GABA is an important inhibitory neurotransmitter that acts within the central nervous system. GABA production in the intestinal area is linked to Lactobacillus which appears to be capable of GABA synthesis. This mechanism is currently under study as neurotransmitter productive probiotics could be used in treating neuro-gastrointestinal disorders [43].
Serotonin has been highly studied as a pathophysiologic background of the gut–brain axis mediated psychiatric symptoms. The production of serotonin in the central nervous system is linked to sleep, mood and appetite and the serotonin produced by entero-chromaffin cells in the intestinal tract has roles in motility and inflammation. However, given the gut–brain axis crosstalk, and the functions this neurotransmitter meets, there is no doubt the serotonin levels via the gut–brain axis are important for modulating mood disorders associated especially with functional digestive symptoms. Animal model studies have demonstrated that serotonin levels are much higher in rich diversified flora mice than germ-free mice [44]. Even more interestingly, germ-free mice have also low hippocampus serotonin levels contrasting with rich tryptophan circulating levels, which is a serotonin precursor. Metabolism of tryptophan is managed by gut flora and being a precursor of serotonin but also of other neurotransmitters involved in neuroendocrine and immune responses. As such, serotonin and probiotic therapy in neuro-gastroenterology are clear options but usage of serotonin directly or its precursor is what studies are debating [45].
Histamine, beyond its immune role, can be produced by enterochromaffin cells and histamine activity within the intestinal tract is linked to intestinal inflammation and luminal integrity. In addition, species of the microbiome are able to also produce histamine. In vitro studies have revealed that Lactobacillus reuteri has histamine producing capacity which decreases the levels of TNF-α, followed by reduction of intestinal inflammation via H2 receptors which raise new hypotheses targeting histamine in inflammatory bowel diseases [46,47].
2.7. Amino Acids and Microbiome
Beyond the critical role of amino acids within the human body, even more important are the essential amino acids that cannot be produced by the body but need to be synthetized from dietary intake These are the branched chain amino acids that are valine, leucine and isoleucine. The microbiome is more specialized in producing branched chain amino acids more than other amino acids. Animal studies have revealed that a restrictive diet and administration of branched chain amino acids increases the bacterial growth of the Bacteroides species more than other species, followed by decreased intestinal inflammatory processes [48,49].
2.8. Microbial Metabolism of Short Chain Fatty Acids
The gut microbiome’s basic roles is the metabolism of complex nutrients and the transformation of complex chemical substances. Nutrients are reduced to simple sugars and fermented down to fatty acids with short chains, with abundance in anaerobic population areas. Short chain fatty acids are acetic, butyric, propionic or lactic acid. Short chain fatty acids also have roles in increasing absorption of vitamins and minerals such as calcium, iron and magnesium and help with glucose and protein liver metabolism. They maintain the integrity of structure and function of the intestinal tract assuring efficient digestive function. Furthermore, they are metabolized as an energy source via the Krebs cycle in the cell environment [50]. Apart from the metabolic and immune roles of short chain fatty acids, influence of the gut–brain axis involves the neuroendocrine axis via acetate modulation of the hypothalamic function, as acetate as well as other acids are capable of reaching the brain by the blood–brain barrier and moderate the activity of neuropeptides responsible for appetite control [51,52]. More relevant and interesting studies conducted on animal models demonstrate that short chain fatty acids could directly influence the brain as they could affect brain areas such as the hippo-campus and striatum, influencing cognitive functions, memory learning and reward-associated behavior (developmental or addictive-like). Moreover, diet supplementation of short chain fatty acids resulted in reduced anhedonia, anxiety and depressive disorders in mice, interestingly accompanied by decreased corticoid receptors in both hypothalamus and colon [53]. Implications of short chain fatty acids in neuropsychiatric disorders are already under heavy research as primary findings offer positive perspectives. High level intra-cerebral, specifically intra-ventricular short chain fatty acids, are supposed to be involved in the pathophysiology of Parkinson’s Disease or even in autistic spectrum disorders and epilepsy [54].
In light of the statements above, future research on all branches of the gut–brain axis should focus primarily on the microbiome and its critical role as a homeostasis mediator between the brain and gastrointestinal environment, as the majority of the mechanisms and reactions of the gut–brain axis are secondary to the gut bacteria’s functions (Figure 1).
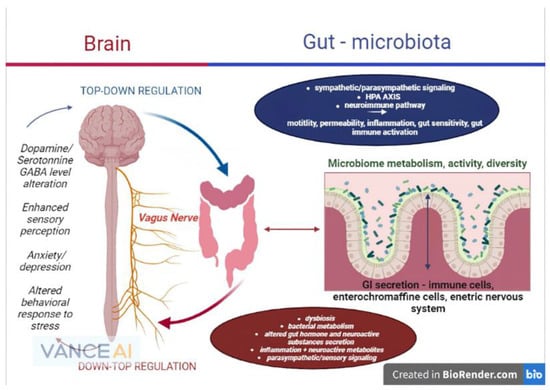
Figure 1. The microbiome–gut–brain axis and its physiopathological interconnections (remake after https://www.frontiersin.org/files/Articles/813204/fmed-09-813204-HTML-r1/image_m/fmed-09-813204-g001.jpg (accessed on 20 September 2022)).
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10112199
This entry is offline, you can click here to edit this entry!
