Antimicrobial peptides (AMPs) are considered a promising therapeutic approach against multi-drug resistant microorganisms. Besides their advantages, there are limitations to be overcome so that these molecules can become market competitive. One of the biggest limitations is proteolytic susceptibility, which could be overcome by structural modifications such as cyclization, especially for helix-constraining strategies. Over the years, many helix stabilization techniques have arisen, such as lactam-bridging, triazole-based, N-alkylation and all-hydrocarbon stapling. All-hydrocarbon stapling takes advantage of modified amino acid residues and olefinic cross-linking to constrain peptide helices. Despite being a well-established strategy and presenting efficient stability results, there are different limitations especially related to toxicity. In this review, recent studies on stapled AMPs for antimicrobial usage are explored with the aim of understanding the future of these molecules as putative antimicrobial agents.
- antimicrobial peptides
- stapling
- all-hydrocarbon stapling
1. Introduction
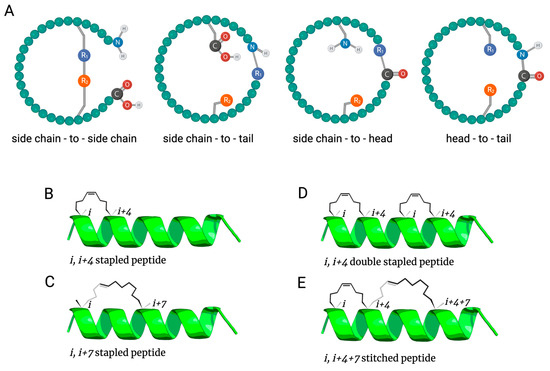
2. Antimicrobial Peptide Stapling
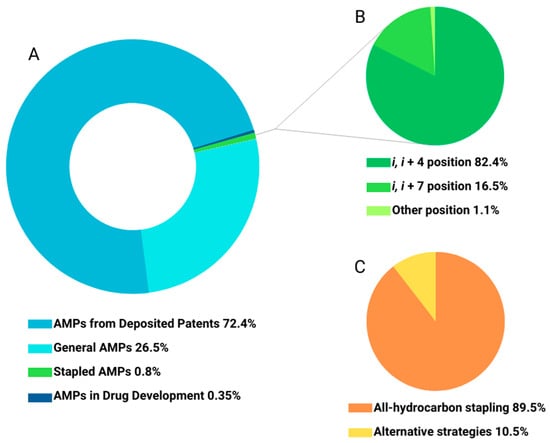
2.1. All-Hydrocarbon Stapling
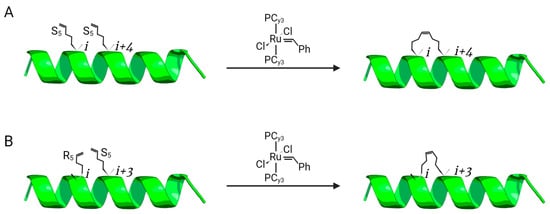
2.2. Alternative Stapling Strategies
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12091400
References
- WHO Global Action Plan on Antimicrobial Resistance. Microbe Mag. 2015, 10, 354–355.
- IACG No Time to Wait: Securing the Future from Drug-Resistant Infections. Rep. Secr.-Gen. United Nations 2019, 54, 113–114.
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.P.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047.
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the Host Defense Peptide Landscape. Front. Chem. 2019, 7, 43.
- de Souza, C.M.; da Silva, Á.P.; Júnior, N.G.O.; Martínez, O.F.; Franco, O.L. Peptides as a Therapeutic Strategy against Klebsiella Pneumoniae. Trends Pharmacol. Sci. 2022, 43, 335–348.
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial Peptides for Therapeutic Use: Obstacles and Realistic Outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472.
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility in Vivo. Biomolecules 2018, 8, 4.
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230.
- Migoń, D.; Neubauer, D.; Kamysz, W. Hydrocarbon Stapled Antimicrobial Peptides. Protein J. 2018, 37, 2–12.
- Tan, P.; Fu, H.; Ma, X. Design, Optimization, and Nanotechnology of Antimicrobial Peptides: From Exploration to Applications. Nano Today 2021, 39, 101229.
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically Modified and Conjugated Antimicrobial Peptides against Superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973.
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical Modifications to Increase the Therapeutic Potential of Antimicrobial Peptides. Peptides 2021, 146, 170666.
- Kapil, S.; Sharma, V. d-Amino Acids in Antimicrobial Peptides: A Potential Approach to Treat and Combat Antimicrobial Resistance. Can. J. Microbiol. 2021, 67, 119–137.
- Manteghi, R.; Pallagi, E.; Olajos, G.; Csóka, I. Pegylation and Formulation Strategy of Anti-Microbial Peptide (AMP) According to the Quality by Design Approach. Eur. J. Pharm. Sci. 2020, 144, 105197.
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target Ther. 2022, 7, 48.
- Andreev, K.; Martynowycz, M.W.; Ivankin, A.; Huang, M.L.; Kuzmenko, I.; Meron, M.; Lin, B.; Kirshenbaum, K.; Gidalevitz, D. Cyclization Improves Membrane Permeation by Antimicrobial Peptoids. Langmuir 2016, 32, 12905–12913.
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The Multifaceted Nature of Antimicrobial Peptides: Current Synthetic Chemistry Approaches and Future Directions. Chem. Soc. Rev. 2021, 50, 7820–7880.
- Khatri, B.; Nuthakki, V.R.; Chatterjee, J. Strategies to Enhance Metabolic Stabilities. Methods Mol. Biol. 2019, 2001, 17–40.
- Skowron, K.J.; Speltz, T.E.; Moore, T.W. Recent Structural Advances in Constrained Helical Peptides. Med. Res. Rev. 2019, 39, 749–770.
- Selvarajan, V.; Tram, N.D.T.; Xu, J.; Ngen, S.T.Y.; Koh, J.-J.; Teo, J.W.P.; Yuen, T.-Y.; Ee, P.L.R. Stapled β-Hairpin Antimicrobial Peptides with Improved Stability and Activity against Drug-Resistant Gram-Negative Bacteria. J. Med. Chem. 2023, 66, 8498–8509.
- Walensky, L.D.; Bird, G.H. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J. Med. Chem. 2014, 57, 6275–6288.
- Schafmeister, C.E.; Po, J.; Verdine, G.L. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J. Am. Chem. Soc. 2000, 122, 5891–5892.
- Kim, Y.W.; Kutchukian, P.S.; Verdine, G.L. Introduction of All-Hydrocarbon i, i + 3 Staples into α-Helices via Ring-Closing Olefin Metathesis. Org. Lett. 2010, 12, 3046–3049.
- Li, X.; Chen, S.; Zhang, W.-D.; Hu, H.-G. Stapled Helical Peptides Bearing Different Anchoring Residues. Chem. Rev. 2020, 120, 10079–10144.
- Wu, C.-L.; Hsueh, J.-Y.; Yip, B.-S.; Chih, Y.-H.; Peng, K.-L.; Cheng, J.-W. Antimicrobial Peptides Display Strong Synergy with Vancomycin Against Vancomycin-Resistant E. faecium, S. Aureus, and Wild-Type E. coli. Int. J. Mol. Sci. 2020, 21, 4578.
- Hirano, M.; Saito, C.; Yokoo, H.; Goto, C.; Kawano, R.; Misawa, T.; Demizu, Y. Development of Antimicrobial Stapled Peptides Based on Magainin 2 Sequence. Molecules 2021, 26, 444.
- Wang, N.; Xie, G.; Liu, C.; Cong, W.; He, S.; Li, Y.; Fan, L.; Hu, H.G. Design, Synthesis, and Antitumor Activities Study of Stapled A4K14-Citropin 1.1 Peptides. Front. Chem. 2020, 8, 616147.
- Bluntzer, M.T.J.; O’Connell, J.; Baker, T.S.; Michel, J.; Hulme, A.N. Designing Stapled Peptides to Inhibit Protein-Protein Interactions: An Analysis of Successes in a Rapidly Changing Field. Pept. Sci. 2021, 113, e24191.
- Wang, C.; Xia, S.; Zhang, P.; Zhang, T.; Wang, W.; Tian, Y.; Meng, G.; Jiang, S.; Liu, K. Discovery of Hydrocarbon-Stapled Short α-Helical Peptides as Promising Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Fusion Inhibitors. J. Med. Chem. 2018, 61, 2018–2026.
- Li, C.; Zhao, N.; An, L.; Dai, Z.; Chen, X.; Yang, F.; You, Q.; Di, B.; Hu, C.; Xu, L. Apoptosis-Inducing Activity of Synthetic Hydrocarbon-Stapled Peptides in H358 Cancer Cells Expressing KRASG12C. Acta Pharm. Sin. B 2021, 11, 2670–2684.
- Yu, J.J.; Zhou, D.D.; Cui, B.; Zhang, C.; Tan, F.W.; Chang, S.; Li, K.; Lv, X.X.; Zhang, X.W.; Shang, S.; et al. Disruption of the EGFR-SQSTM1 Interaction by a Stapled Peptide Suppresses Lung Cancer via Activating Autophagy and Inhibiting EGFR Signaling. Cancer Lett. 2020, 474, 23–35.
- Kim, M.I.; Pham, T.K.; Kim, D.; Park, M.; Kim, B.O.; Cho, Y.H.; Kim, Y.W.; Lee, C. Identification of Brevinin-1EMa-Derived Stapled Peptides as Broad-Spectrum Virus Entry Blockers. Virology 2021, 561, 6–16.
- Curreli, F.; Victor, S.M.B.; Ahmed, S.; Drelich, A.; Tong, X.; Tseng, C.-T.K.; Hillyer, C.D.; Debnath, A.K. Stapled Peptides Based on Human Angiotensin-Converting Enzyme 2 (ACE2) Potently Inhibit SARS-CoV-2 Infection In Vitro. mBio 2020, 11, e02451-20.
- Maas, M.N.; Hintzen, J.C.J.; Löffler, P.M.G.; Mecinović, J. Targeting SARS-CoV-2 Spike Protein by Stapled HACE2 Peptides. Chem. Commun. 2021, 57, 3283–3286.
- de Campos, L.J.; Palermo, N.Y.; Conda-Sheridan, M. Targeting SARS-CoV-2 Receptor Binding Domain with Stapled Peptides: AnIn SilicoStudy. J. Phys. Chem. B 2021, 125, 6572–6586.
- Stone, T.A.; Cole, G.B.; Nguyen, H.Q.; Sharpe, S.; Deber, C.M. Influence of Hydrocarbon-Stapling on Membrane Interactions of Synthetic Antimicrobial Peptides. Bioorg. Med. Chem. 2018, 26, 1189–1196.
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An Enhanced Comprehensive Data Repository of Antimicrobial Peptides. Nucleic Acids Res. 2021, 50, D488–D496.
- Kim, Y.-W.; Grossmann, T.N.; Verdine, G.L. Synthesis of All-Hydrocarbon Stapled α-Helical Peptides by Ring-Closing Olefin Metathesis. Nat. Protoc. 2011, 6, 761–771.
- Tan, Y.S.; Lane, D.P.; Verma, C.S. Stapled Peptide Design: Principles and Roles of Computation. Drug Discov. Today 2016, 21, 1642–1653.
- Bird, G.H.; Madani, N.; Perry, A.F.; Princiotto, A.M.; Supko, J.G.; He, X.; Gavathiotis, E.; Sodroski, J.G.; Walensky, L.D. Hydrocarbon Double-Stapling Remedies the Proteolytic Instability of a Lengthy Peptide Therapeutic. Proc. Natl. Acad. Sci. USA 2010, 107, 14093–14098.
- Hilinski, G.J.; Kim, Y.W.; Hong, J.; Kutchukian, P.S.; Crenshaw, C.M.; Berkovitch, S.S.; Chang, A.; Ham, S.; Verdine, G.L. Stitched α-Helical Peptides via Bis Ring-Closing Metathesis. J. Am. Chem. Soc. 2014, 136, 12314–12322.
- Zheng, M.; Wang, R.; Chen, S.; Zou, Y.; Yan, L.; Zhao, L.; Li, X. Design, Synthesis and Antifungal Activity of Stapled Aurein1.2 Peptides. Antibiotics 2021, 10, 956.
- Luong, H.X.; Kim, D.H.; Lee, B.J.; Kim, Y.W. Antimicrobial Activity and Stability of Stapled Helices of Polybia-MP1. Arch. Pharm. Res. 2017, 40, 1414–1419.
- Crawford, M.A.; Ward, A.E.; Gray, V.; Bailer, P.; Fisher, D.J.; Kubicka, E.; Cui, Z.; Luo, Q.; Gray, M.C.; Criss, A.K.; et al. Disparate Regions of the Human Chemokine CXCL10 Exhibit Broad-Spectrum Antimicrobial Activity against Biodefense and Antibiotic-Resistant Bacterial Pathogens. ACS Infect. Dis. 2023, 9, 122–139.
- Souza, B.M.; Mendes, M.A.; Santos, L.D.; Marques, M.R.; César, L.M.M.; Almeida, R.N.A.; Pagnocca, F.C.; Konno, K.; Palma, M.S. Structural and Functional Characterization of Two Novel Peptide Toxins Isolated from the Venom of the Social Wasp Polybia Paulista. Peptides 2005, 26, 2157–2164.
- Dinh, T.T.T.; Kim, D.H.; Luong, H.X.; Lee, B.J.; Kim, Y.W. Antimicrobial Activity of Doubly-Stapled Alanine/Lysine-Based Peptides. Bioorg. Med. Chem. Lett. 2015, 25, 4016–4019.
- Stark, M.; Liu, L.-P.; Deber, C.M. Cationic Hydrophobic Peptides with Antimicrobial Activity. Antimicrob. Agents Chemother. 2002, 46, 3585–3590.
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The Antibiotic and Anticancer Active Aurein Peptides from the Australian Bell Frogs Litoria Aurea and Litoria Raniformis. Eur. J. Biochem. 2000, 267, 5330–5341.
- Liu, B.; Zhang, W.; Gou, S.; Huang, H.; Yao, J.; Yang, Z.; Liu, H.; Zhong, C.; Liu, B.; Ni, J.; et al. Intramolecular Cyclization of the Antimicrobial Peptide Polybia-MPI with Triazole Stapling: Influence on Stability and Bioactivity. J. Pept. Sci. 2017, 23, 824–832.
- Li, H.; Hu, Y.; Pu, Q.; He, T.; Zhang, Q.; Wu, W.; Xia, X.; Zhang, J. Novel Stapling by Lysine Tethering Provides Stable and Low Hemolytic Cationic Antimicrobial Peptides. J. Med. Chem. 2020, 63, 4081–4089.
