Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Gemini surfactants are a modern variety of surfactants with unique properties and a very wide range of potential applications. Hexamethylene-1,6-bis(N-dodecyl-N,N-dimethylammonium bromide) is one such representative compound that is a better alternative to a single analogue. It shows excellent surface, antimicrobial, and anticorrosion properties. With a highly efficient synthetic method and a good ecological profile, it is a potential candidate for numerous applications, including biomedical applications.
- gemini surfactants
- 12-6-12
- synthesis
- properties
- antimicrobial activity
- applications
1. Introduction
Interactions at the interface are of fundamental importance in chemistry, physics, and biology. The thermodynamic parameters of these interactions can be modulated using surfactants. Owing to their amphiphilic structure, which comprises a hydrophilic and hydrophobic part, surfactants decrease the surface tension or interfacial tension between two liquids, a liquid and gas, or a liquid and solid. Hence, they can act as wetting, dispersing, and emulsifying agents. These properties enable the production of a large number of products required in household chemistry and cosmetic, pharmaceutical, agrochemical, petrochemical, textile, and paper industries.
The dynamic development of surfactant chemistry is a continuation of what was initiated by nature, which created the biosurfactants necessary for the functioning of living organisms. Biosurfactants usually refer to surfactants of microbial origin and comprise lecithin, rhamnolipids, sophorolipids, and emulsan [1][2]. Biosurfactants, in addition to their intrinsic role, have a wide range of technical applications. For instance, they can solubilise hydrocarbon contaminants and can be used in enhanced oil recovery [3][4]. Owing to their low toxicity and biodegradability, biosurfactants are extremely valuable products from the viewpoint of environmental protection. The global market size for biosurfactants reached a value of more than USD 2.33 billion in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 5.8% between 2023 and 2028, reaching a projected value of USD 3.27 billion by 2027 [5][6]. Unfortunately, the number of available biosurfactants as well as the range of their applications does not meet the requirements expected from surfactants.
Currently, the widest groups of surfactants that meet the application requirements are nonionic, anionic, cationic, and amphoteric synthetic surfactants. The global demand for these surfactants, including soaps, exceeds 20 million tons per year [6]. The global surfactant market stood at a value of approximately USD 41.84 billion in 2022. The market is further expected to grow in the forecast period 2023–2030 at a CAGR of 4.6% to reach a value of USD 59.95 billion by 2030 [6]. However, the large volume of surfactants disposed into the environment, despite the wastewater treatment processes, is a serious burden and threat to the environment. Thus, owing to the increasing demand for surfactants, the development of new and more effective surfactants is extremely important.
Cationic gemini surfactants have emerged as a result of the research conducted in this field over the last several years. Gemini surfactants are compounds that are composed of two hydrophilic head groups and two hydrophobic tails linked by a spacer at the head groups or close to them (Figure 1). The spacers can be flexible (methylenes) or rigid (aromatic structures). The type of spacer (short or long, hydrophilic or hydrophobic) influences the shape of the micelles. The neutral charge of the molecule is retained in the presence of organic or inorganic counterions [7][8][9].
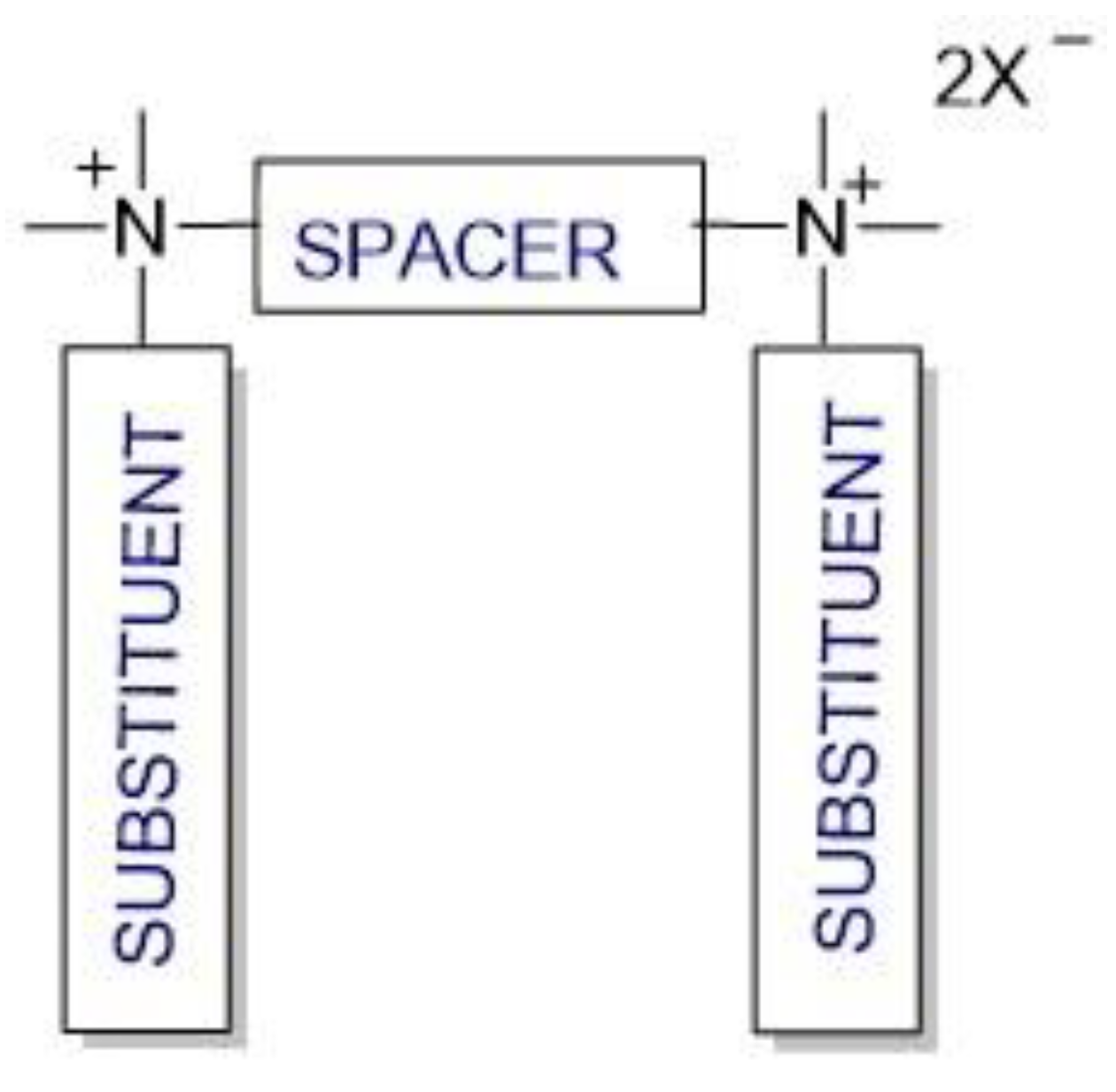
Figure 1. Structure of gemini surfactants.
The critical micelle concentration (CMC), surface tension (γ), and minimal inhibitory concentration (MIC) of gemini surfactants are a dozen times lower than those of monomeric surfactants. The unique properties of gemini surfactants with a wide range of hydrophilic–lipophilic balance (HLB) render them a very useful and innovative material in chemistry (e.g., for corrosion inhibition, micellar catalysis, nanoparticle synthesis, preparation of supramolecular solvents, nanoemulsion preparation, and synthesis of precisely defined polymers) [10][11][12][13][14], medicine (as biocides, drug carriers, and capping agents for metal nanoparticles with biocidal properties or for preparing nonviral gene delivery systems and inducing protein conformational changes) [15][16][17][18][19][20], and optoelectronics (through a spatial network of well-dispersed molecules) [7]. Gemini surfactants are a modern solution for all areas that need surfactants, including households, detergents, personal care, institutional and industrial cleaning, food processing, plastics, paints and coatings, oilfield chemicals, petrochemistry, agricultural chemicals, adhesives, and textiles [20].
2. Structure and Synthesis
12-6-12 consists of two N-dodecyl-N,N-dimethylammonium units connected with a chain of six methylene groups as a spacer. Bromine ions are present as counterions (Figure 2). This compound has been classified under chemical abstracts service (CAS) number 18507-15-8 and is a dimeric analogue of N-dodecyl-N,N,N-trimethylammonium bromide (DTAB), which is commonly used as a microbiocide.
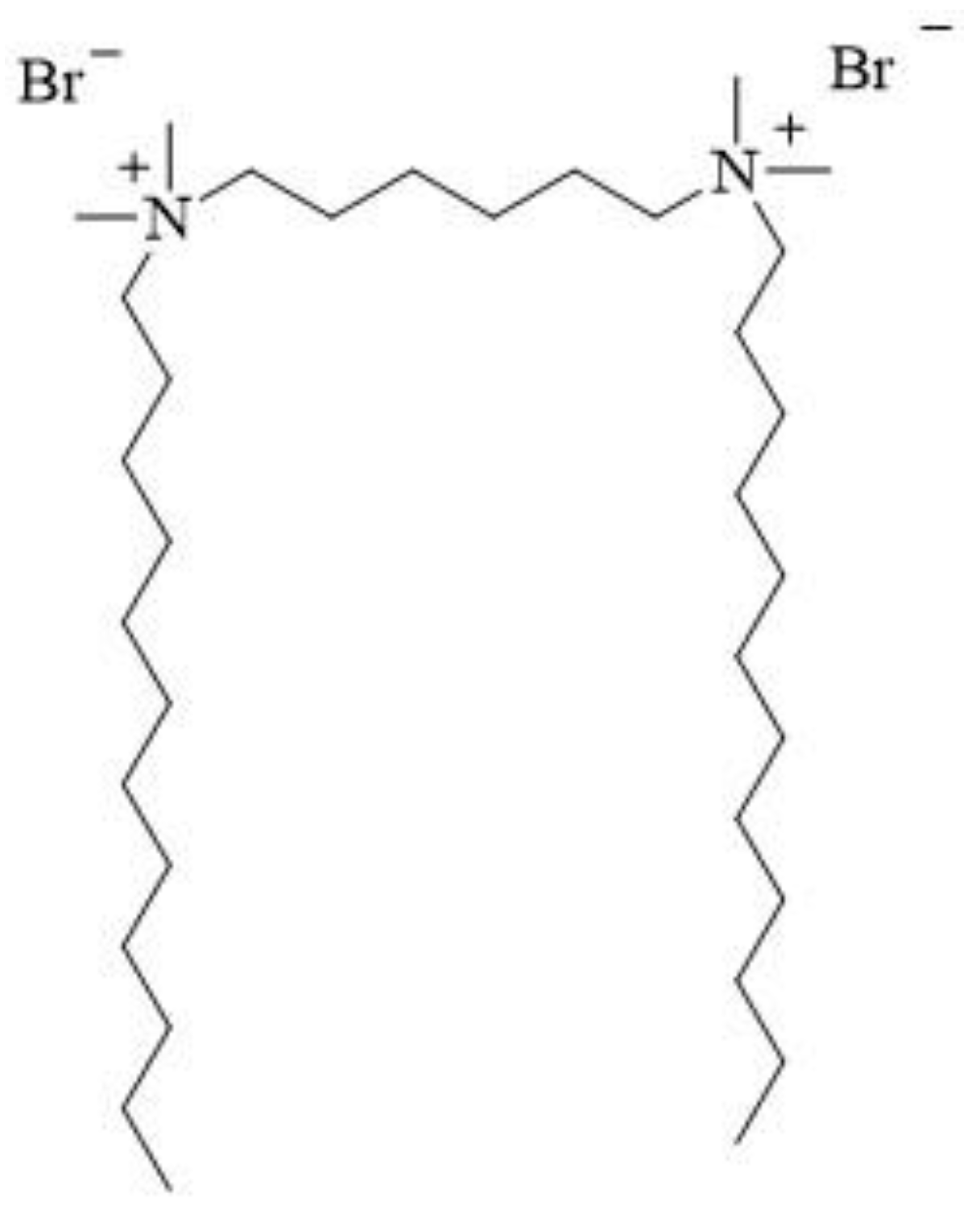
Figure 2. Chemical structure of 12-6-12.
12-6-12 is one of the first compound to be classified as a double quaternary ammonium salt and defined as a gemini surfactant. This compound is synthesised via the quaternisation of amines, a process referred to as the Menschutkin reaction:
-
alkylation of hexamethylene-bis(N,N-dimethylamine) with 1-dodecylbromide (Figure 3a);
-
linking of N-dodecyl-N,N-dimethylamine with 1,6-dibromohexane (Figure 3b).
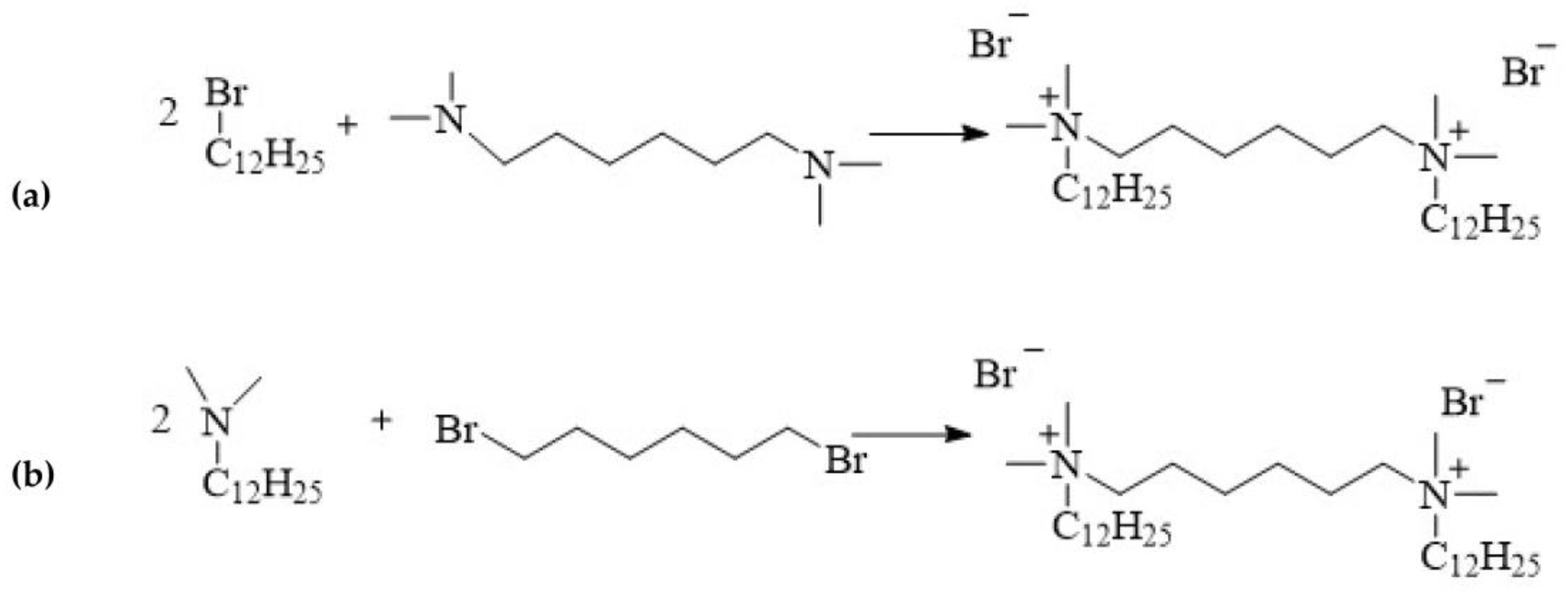
Figure 3. Synthesis of 12-6-12.
The synthesis of 12-6-12 was first reported in 1968 by Sindenko et al. [21]. Stoichiometric amounts of dodecylbromide and hexamethylene-bis(N,N-dimethylamine) were reacted in ethanol for the synthesis (yield 70%) [21]. Regardless of the synthetic pathway, the quaternisation reaction always follows the SN2 nucleophilic substitution mechanism. The rate of the reaction depends on the concentrations of both reagents, although there are reports of the use of excess amine [22][23][24][25] or bromide [26][27][28]. Typically, polar solvents such as alcohols (methanol [27][29], ethanol [30][31], isopropanol [32]), acetone [19][23][33], or acetonitrile [22][24][34] are used in the synthesis of 12-6-12. These reactions occur at the boiling point of the solvent. The type of solvent used determines the reaction time because SN2 reactions are the fastest in polar aprotic solvents such as acetonitrile. Replacing ethanol with acetonitrile reduces the reaction time from 24 to 5 h [25][35]. 12-6-12 can also be synthesised in solvent mixtures such as the acetonitrile/toluene mixture [36]. The reaction time can be shortened using microwave radiation [37]. However, the most economical and ecological approach is to synthesise 12-6-12 under stoichiometric conditions at room temperature without a solvent [38]. In this case, good yields of over 90% can be achieved using small amounts of reagents over a reaction time of 0.5 h [38]. Solvent-free synthesis can also be easily carried out on a large scale.
To obtain pure 12-6-12, the crude product can be crystallised from acetonitrile [35] or from dichloromethane–diethyl ether [39], acetone–methanol [40][41], ethanol–ethyl acetate [23], ethanol–diethyl ether [24], acetone–ethanol [21], and acetone–ethyl acetate [25] mixtures.
3. Applications
Surfactants are ubiquitous, being key components in a diverse range of complex industrial processes and utilitarian products such as dispersants, solubilisers, emulsifiers, demulsifiers, foaming agents, wetting agents, disinfectants, corrosion inhibitors, antistatic agents, and viscosity modifiers. In the last few decades, significant efforts have been made for the synthesis of new gemini surfactants, fuelled by their remarkably improved physicochemical properties that can be achieved by the modification of structural factors [42]. Cationic gemini surfactants have wide applications due to their excellent surface activity [7][20]:
-
in foaming, agrichemical spreading aids, and cleaning;
-
from industrial to personal care applications;
-
phase transfer catalyst;
-
bleach activator;
-
as hair conditioners and fabric softeners.
12-6-12 was first described 55 years ago, but the increased application interest of this compound can be defined in the 21st century. 12-6-12 can be used in several practical applications (Figure 4).
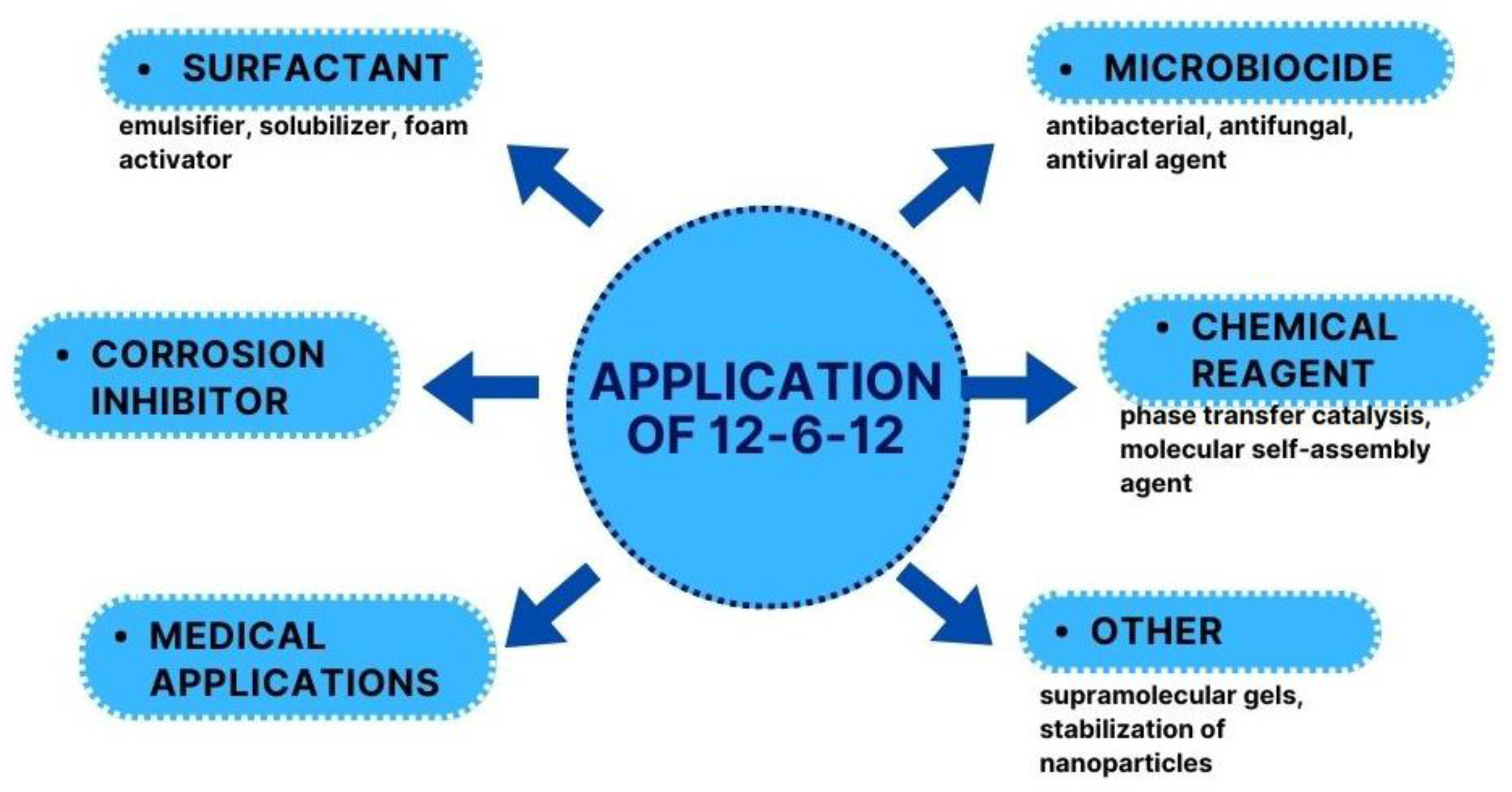
Figure 4. Applications of 12-6-12.
12-6-12 is characterised by excellent surface, antimicrobial, and anticorrosion properties. As a compound that can be obtained economically and ecologically, it is an ideal product that has many industrial applications, including bioapplications (Table 1).
Table 1. Potential bioapplications of 12-6-12.
| Application | References |
|---|---|
| Enhanced drug delivery | [43][44] |
| Drug carrier | [45] |
| Stabilization of drugs | [46] |
| Detection of DNA | [33] |
| Nonviral gene delivery agent | [47][48][49][50] |
| Eradication of biofilm | [51][52] |
| Filtering nonwovens | [53] |
| Bioactive cellulose products | [54][55] |
| Addition to DNA filtration | [56] |
Moreover, 12-6-12 can be used in chemical synthesis for the preparation of supramolecular gels [57] and as a nanoparticle stabiliser [28][41][58], an interfacial transfer catalyst [30][31][59][60][61], and a molecular self-assembly agent [62].
In conclusion, quaternary ammonium gemini surfactants are known for their multifunctional utility properties such as antimicrobial, surfactant, and anticorrosion properties. 12-6-12 has all the above mentioned properties of gemini surfactants and can be obtained in a one-step reaction, which is an indisputable and economically justified advantage over other gemini surfactants. Compared to its monochain counterpart, it shows better surface, antimicrobial, and anticorrosion activity and is characterised by a lower toxicity. The use of this compound in concentrations much lower than DTAB while providing the same utility effect makes 12-6-12 a candidate in many applications. Initially, this compound was used in applications as typical quaternary ammonium salts as an interfacial transfer catalyst. Currently, this compound finds potential applications in many different areas of life. Particularly noteworthy is the testing of this compound in biomedical applications. Due to its anticorrosive, biocidal, dispersing, and detergent properties, 12-6-12 can be used as an additive to car fuels and as an anticorrosion and antimicrobial agent in oil pipelines, smart anticorrosion coatings, etc. Hence, the possibility of producing it in large quantities in a one-step, waste-free synthesis is a huge advantage over other gemini surfactants.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28176336
References
- Roy, A. A Review on the Biosurfactants: Properties, Types and its Applications. J. Fundam. Renew. Energy Appl. 2018, 8, 248.
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662.
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial Biosurfactant as an Alternate to Chemical Surfactants for Application in Cosmetics Industries in Personal and Skin Care Products: A Critical Review. BioMed Res. Int. 2023, 2023, 2375223.
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391.
- Global Biosurfactants Market: By Type: Glycolipids, Lipopeptides, Fatty Acids, Polymerics, Others; By Application: Emulsifiers, Humectants, Preserving Agents, By End Use: Detergents, Personal Care, Food Processing, Agrochemicals; Regional Analysis; Competitive Landscape; Key Trends and Developments in the Market; 2023–2028. Available online: https://www.expertmarketresearch.com/reports/biosurfactants-market (accessed on 29 August 2023).
- Surfactants Market—Global Industry Assessment & Forecast. Available online: https://www.vantagemarketresearch.com/industry-report/surfactants-market-1671 (accessed on 29 August 2023).
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3325-4.
- Menger, F.M.; Littau, C.A. Gemini-surfactants: Synthesis and properties. J. Am. Chem. Soc. 1991, 113, 1451–1452.
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920.
- Brycki, B.; Szulc, A. Gemini surfactants as corrosion inhibitors. A review. J. Mol. Liq. 2021, 344, 117686.
- Li, D.; Fang, W.; Feng, Y.; Geng, Q.; Song, M. Stability properties of water-based gold and silver nanofluids stabilized by cationic gemini surfactants. J. Taiwan Inst. Chem. Eng. 2019, 97, 458–465.
- Feizi, N.; Yamini, Y.; Moradi, M.; Karimi, M.; Salamat, Q.; Amanzadeh, H. A new generation of nano-structured supramolecular solvents based on propanol/gemini surfactant for liquid phase microextraction. Anal. Chim. Acta 2017, 953, 1–9.
- Yadav, S.K.; Parikh, K.; Kumar, S. Solubilization potentials of single and mixed oppositely charged gemini surfactants: A case of polycyclic aromatic hydrocarbons. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 47–55.
- Ghosh, K.K.; Kolay, S.; Bal, S.; Satnami, M.L.; Quagliotto, P.; Dafonte, P.R. Effect of cationic gemini surfactants on the hydrolysis of carboxylate and phosphate esters using hydroxamate ions. Colloid Polym. Sci. 2008, 286, 293–303.
- Kirby, A.J.; Camilleri, P.; Engberts, J.B.F.N.; Feiters, M.C.; Nolte, R.J.M.; Söderman, O.; Bergsma, M.; Bell, P.C.; Fielden, M.L.; García Rodríguez, C.L.; et al. Gemini Surfactants: New Synthetic Vectors for Gene Transfection. Angew. Chem. Int. Ed. 2003, 42, 1448–1457.
- Akram, M.; Ansari, F.; Bhat, I.A.; Din, K.U. Probing interaction of bovine serum albumin (BSA) with the biodegradable version of cationic gemini surfactants. J. Mol. Liq. 2019, 276, 519–528.
- Akram, M.; Ansari, F.; Bhat, I.A.; Chaturvedi, S.K.; Khan, R.H.; Din, K.U. Analyzing the interaction between porcine serum albumin (PSA) and ester-functionalized cationic gemini surfactants. Process Biochem. 2017, 63, 145–153.
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 2022, 299, 102581.
- Mirgorodskaya, A.B.; Ya Zakharova, L.; Khairutdinova, E.I.; Lukashenko, S.S.; Sinyashin, O.G. Supramolecular systems based on gemini surfactants for enhancing solubility of spectral probes and drugs in aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 33–42.
- Kumar, N.; Tyagi, R. Industrial Applications of Dimeric Surfactants: A Review. J. Dispers. Sci. Technol. 2014, 35, 205–214.
- Sidenko, Z.S.; Limanov, V.E.; Skvortsova, E.K.; Dziomko, V.M. Synthesis and antibacterial activity of several bisammonium compounds. Pharm. Chem. J. 1968, 2, 247–250.
- McLachlan, A.; Singh, K.; Piggott, E.; McAlduff, M.; MacLennan, S.; Sandre, V.; Reid, T.; Marangoni, D.G. m-s-m Cationic Gemini and Zwitterionic Surfactants–Spacer-Dependent Synergistic Interactions. J. Phys. Chem. B 2019, 123, 1855–1868.
- Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R.K. Micellization of Cationic Gemini Surfactants with Various Counterions and Their Interaction with DNA in Aqueous Solution. J. Phys. Chem. B 2004, 108, 15385–15391.
- Mivehi, L.; Bordes, R.; Holmberg, K. Adsorption of Cationic Gemini Surfactants at Solid Surfaces Studied by QCM-D and SPR: Effect of the Rigidity of the Spacer. Langmuir 2011, 27, 7549–7557.
- Zana, R.; Benrraou, M.; Rueff, R. Alkanediyl-.alpha.,.omega.-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 1991, 7, 1072–1075.
- Szymaniak, D.; Maćkowiak, A.; Ciarka, K.; Praczyk, T.; Marcinkowska, K.; Pernak, J. Synthesis and Characterization of Double-Salt Herbicidal Ionic Liquids Comprising both 4-Chloro-2-methylphenoxyacetate and trans-Cinnamate Anions. ChemPlusChem 2020, 85, 2281–2289.
- Junior, P.B.S.; Tiera, V.A.O.; Tiera, M.J. A fluorescence probe study of gemini surfactants in aqueous solution: A comparison between n-2-n and n-6-n series of the alkanediyl-α,ω-bis (dimethylalkylammonium bromides). Eclet. Quím. 2007, 32, 47–54.
- Takács, D.; Péter, T.; Vargáné Árok, Z.; Katana, B.; Papović, S.; Gadzuric, S.; Vraneš, M.; Szilágyi, I. Structure–Stability Relationship in Aqueous Colloids of Latex Particles and Gemini Surfactants. J. Phys. Chem. B 2022, 126, 9095–9104.
- Imam, T.; Devinsky, F.; Lacko, I.; Mlynarčík, D.; Krasnec, L. Preparation and antimicrobial activity of some new bisquaternary ammonium salts. Pharmazie 1983, 38, 308–310.
- Esen, I.; Yolacan, C.; Aydogan, F. Long Chain Dicationic Phase Transfer Catalysts in the Condensation Reactions of Aromatic Aldehydes in Water Under Ultrasonic Effect. Bull. Korean Chem. Soc. 2010, 31, 2289–2292.
- Öge, A.; MaviŞ, M.E.; Yolaçan, Ç.; Aydoğan, F. Solvent-free Michael addition of 2-cyclohexenone under ultrasonic irradiation in the presence of long chain dicationic ammonium salts. Turk. J. Chem. 2012, 36, 137–146.
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169.
- Zou, Q.-C.; Zhang, J.-Z.; Chai, S.-G. Resonance light scattering method for the determination of DNA with cationic methacrylate based polymer nanoparticle probes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 437–443.
- Lee, H.I.; Pak, C.; Yi, S.H.; Shon, J.K.; Kim, S.S.; So, B.G.; Chang, H.; Yie, J.E.; Kwon, Y.-U.; Kim, J.M. Systematic phase control of periodic mesoporous organosilicas using Gemini surfactants. J. Mater. Chem. 2005, 15, 4711.
- Brycki, B.; Kowalczyk, I.; Kozirog, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium Bromides). Molecules 2011, 16, 319–335.
- Wang, H.; Zhao, W.; Zhou, L.; Wang, J.; Liu, L.; Wang, S.; Wang, Y. Soft Particles of Gemini Surfactant/Conjugated Polymer for Enhanced Anticancer Activity of Chemotherapeutics. ACS Appl. Mater. Interfaces 2018, 10, 37–41.
- Singer, O.M.; Campbell, J.W.; Hoare, J.G.; Masuda, J.D.; Marangoni, G.; Singer, R.D. Improved Green Synthesis and Crystal Structures of Symmetrical Cationic Gemini Surfactants. ACS Omega 2022, 7, 35326–35330.
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, spectroscopic studies, aggregation and surface behavior of hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide). J. Mol. Liq. 2016, 221, 1086–1096.
- Calas, M.; Ancelin, M.L.; Cordina, G.; Portefaix, P.; Piquet, G.; Vidal-Sailhan, V.; Vial, H. Antimalarial Activity of Compounds Interfering with Plasmodium falciparum Phospholipid Metabolism: Comparison between Mono- and Bisquaternary Ammonium Salts. J. Med. Chem. 2000, 43, 505–516.
- Devinsky, F.; Pisarcik, M.; Lacko, I. Hydrodynamic size of DNA/cationic gemini surfactant complex as a function of surfactant structure. Gen. Physiol. Biophys. 2009, 28, 160–167.
- Pisárčik, M.; Jampílek, J.; Lukáč, M.; Horáková, R.; Devínsky, F.; Bukovský, M.; Kalina, M.; Tkacz, J.; Opravil, T. Silver Nanoparticles Stabilised by Cationic Gemini Surfactants with Variable Spacer Length. Molecules 2017, 22, 1794.
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68.
- Almeida, J.A.S.; Faneca, H.; Carvalho, R.A.; Marques, E.F.; Pais, A.A.C.C. Dicationic Alkylammonium Bromide Gemini Surfactants. Membrane Perturbation and Skin Irritation. PLoS ONE 2011, 6, e26965.
- Silva, S.M.C.; Sousa, J.J.S.; Marques, E.F.; Pais, A.A.C.C.; Michniak-Kohn, B.B. Structure Activity Relationships in Alkylammonium C12-Gemini Surfactants Used as Dermal Permeation Enhancers. AAPS J. 2013, 15, 1119–1127.
- Yang, J.; Yun, L.; Zhao, G.; Zhang, F.; Chen, Y.; Wang, C. Fabrication of pH-responsive system based on cationic gemini surfactant/sodium octanedioate and its application on controlled release of paclitaxel. Colloids Surf. A 2018, 539, 101–108.
- Wang, M.; Wu, C.; Tang, Y.; Fan, Y.; Han, Y.; Wang, Y. Interactions of cationic trimeric, gemini and monomeric surfactants with trianionic curcumin in aqueous solution. Soft Matter 2014, 10, 3432.
- Buse, J.; Badea, I.; Verrall, R.E.; El-Aneed, A. Tandem Mass Spectrometric Analysis of the Novel Gemini Surfactant Nanoparticle Families G12-s and G18:1-s. Spectrosc. Lett. 2010, 43, 447–457.
- Wettig, S.D.; Deubry, R.; Akbar, J.; Kaur, T.; Wang, H.; Sheinin, T.; Joseph, J.W.; Slavcev, R.A. Thermodynamic investigation of the binding of dissymmetric pyrenyl-gemini surfactants to DNA. Phys. Chem. Chem. Phys. 2010, 12, 4821–4826.
- Falsini, S.; Di Cola, E.; In, M.; Giordani, M.; Borocci, S.; Ristori, S. Complexation of short ds RNA/DNA oligonucleotides with Gemini micelles: A time resolved SAXS and computational study. Phys. Chem. Chem. Phys. 2017, 19, 3046–3055.
- Acosta-Martínez, D.R.; Rodríguez-Velázquez, E.; Araiza-Verduzco, F.; Taboada, P.; Prieto, G.; Rivero, I.A.; Pina-Luis, G.; Alatorre-Meda, M. Bis-quaternary ammonium gemini surfactants for gene therapy: Effects of the spacer hydrophobicity on the DNA complexation and biological activity. Colloids Surf. B Biointerfaces 2020, 189, 110817.
- Koziróg, A.; Kręgiel, D.; Brycki, B. Action of Monomeric/Gemini Surfactants on Free Cells and Biofilm of Asaia lannensis. Molecules 2017, 22, 2036.
- Koziróg, A.; Otlewska, A.; Brycki, B. Viability, Enzymatic and Protein Profiles of Pseudomonas aeruginosa Biofilm and Planktonic Cells after Monomeric/Gemini Surfactant Treatment. Molecules 2018, 23, 1294.
- Majchrzycka, K.; Okrasa, M.; Szulc, J.; Brycki, B.; Gutarowska, B. Time-Dependent Antimicrobial Activity of Filtering Nonwovens with Gemini Surfactant-Based Biocides. Molecules 2017, 22, 1620.
- Koziróg, A.; Brycki, B.; Olejnik, K.; Wysocka-Robak, A.; Dębska-Winkler, P. Cellulose products modified with monomeric and gemini surfactants: Antimicrobial aspects. Cellulose 2019, 26, 5559–5570.
- Wietecha, J.; Kopania, E.; Ciechańska, D.; Wiśniewska-Wrona, M.; Gruchała, B.; Data, M.; Brycki, B.; Kowalczyk, I. Kompozycja sanityzująca do wyrobów papierniczych. Patent PL 232698B1, 1 August 2016.
- Akbari, S.; Pakpour, S.; Shabanloo, R.; Mirsalehi, M.; Ko, F.; Sadeghzadeh Milani, A.; Brycki, B.; Pyrzyński, K. Active electrospun layer for DNA filteration. Patent WO2022157547A1, 23 January 2021.
- Wang, H.; He, L.; Brycki, B.E.; Kowalczyk, I.H.; Kuliszewska, E.; Yang, Y. Electrochemical characterization of the hydrophobic microenvironment within gemini surfactant micellar-hybridized supramolecular gels. Electrochim. Acta 2013, 90, 326–331.
- Gustafsson, H.; Isaksson, S.; Altskär, A.; Holmberg, K. Mesoporous silica nanoparticles with controllable morphology prepared from oil-in-water emulsions. J. Colloid Interface Sci. 2016, 467, 253–260.
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.S.; Rizvanova, L.Z.; Konovalov, A.I. Geminal alkylammonium surfactants: Aggregation properties and catalytic activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631.
- Mirgorodskaya, A.B.; Kudryavtseva, L.A. Aqueous solutions of geminal alkylammonium surfactants as a medium for reactions of long-chain amines. Russ. J. Gen. Chem. 2009, 79, 42–48.
- Mirgorodskaya, A.B.; Yackevich, E.I.; Valeeva, F.G.; Pankratov, V.A.; Zakharova, L.Y. Solubilizing and catalytic properties of supramolecular systems based on gemini surfactants. Russ. Chem. Bull. 2014, 63, 82–87.
- Deng, S.; Zhao, J.; Wen, Z. Self-Assembly of Quaternary Ammonium Gemini Surfactants in Cyclohexane upon Reinforcement by Simple Counterions. RSC Adv. 2018, 8, 18880–18888.
This entry is offline, you can click here to edit this entry!
