Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
There are several Amazonian plant species with potential pharmacological validation for the treatment of acute kidney injury, a condition in which the kidneys are unable to adequately filter the blood, resulting in the accumulation of toxins and waste in the body.
- Amazonian traditional medicine
- phytotherapy
- oxidative stress
- nephroprotection
1. Banisteriopsis caapi (Spruce ex Griseb.) Morton
Banisteriopsis caapi (Spruce ex Griseb.) Morton is a woody liana, common around the Amazon Basin, belonging to the kingdom Plantae, class Equisetopsida C. Agardh, order Malpighiales Juss. Former Bercht. & J. Presl, family Malpighiaceae Juss., genus Banisteriopsis CB Rob. and species Banisteriopsis caapi (Spruce ex Griseb.) Morton, which has broad ethnopharmacological use by the Amazonian people [1]. It is a species used as the main ingredient of the hallucinogenic drink, called ayahuasca, consumed by religious groups in Brazil to treat various ailments [2]. It is consumed for its hallucinogenic properties, which have been known by many of the indigenous people of the Amazon for centuries [3].
Some species of the Malpighiaceae family are known to produce alkaloids, among them, B. caapi (Spruce ex Griseb.) Morton. Harmine, one of the main alkaloids found in B. caapi (Spruce ex Griseb.) Morton, a plant widely consumed in the ayahuasca drink It is a β-carboline alkaloid widely disseminated due to its monoaminoxidase (MAO) inhibitory activity. As seen in the studies by Samoylenko et al. [4], harmine and harmaline (obtained from aqueous extracts of fresh and dried branches of B. caapi (Spruce ex Griseb.) Morton), have potent effects on MAO inhibitory and antioxidant activity. In addition, strong antioxidant activity for inhibition of cellular reactive oxygen species (ROS) generation by phorbol-12-myristate-13-acetate (PMA) has also been observed.
The effects of harmine against nicotine-induced damage in mouse kidneys were detailed by Salahshoor et al. [5]. In the study, administration of harmine to nicotine-treated animals significantly improved renal malondialdehyde (MDA), blood urea nitrogen (BUN), creatinine, and nitrite oxide levels. It increased the number of glomeruli and the level of power tissue ferric reducer/antioxidant (FRAP) compared to the nicotine group (p < 0.05), Table 1.
Table 1. Phytocompounds from Amazonian plant species and their pharmacological activity.
| Species | Parts Used | Isolated or Characterized Constituents | Pharmacological Activity |
|
|---|---|---|---|---|
| Banisteriopsis caapi (Spruce ex Griseb.) Morton | Stem | Harmine (1), harmaline (2) [4], tetrahydroharmine (3), and harmalinic acid (4) [6] | Analgesic [7], hallucinogen [8], anesthetic [9], antidiabetic [10], anticancerogenic [11], nephroprotective, diuretic [5] | |
| Peganum harmala L. | Seeds | Harmol (5), harmalol (6), harmine (1), and harmaline (2) [12] | Antioxidant, nephroprotective, anti-inflammatory, anti-apoptotic [12] | |
| Passiflora edulis Sims | Fruit peel, leaves, flowers, seeds | Orientin (7) and isoorientin (8) [13] | Anxiolytic, sedative, neuropathic pain [14], anticonvulsant [15], cognitive function and degenerative diseases [16], antioxidant action, antitumor action, hypoglycemic action, obesity, insomnia, nephroprotector [17] | |
| Annona muricata L. | Leaves | Acetogenin (9) [18], δ-Cadinene (10), and α-Muurolene (11) [19] | Anticancerogenic, hepatoprotective, neurotoxic, antinociceptive, antiulcerative, chemopreventive, nephroprotective [20] | |
| Uncaria tomentosa (Willd.) DC. | Stem | Uncarine F (12), speciophylline (13), and mitraphylline (14) [21] | Antioxidant and immunomodulator, anti-inflammatory, analgesic, anticancer, and diuretic [22] | |
| Hymenaea courbaril L. | Stem and leaves | Fisetin (15), cyclosativene (16), caryophyllene (17), and α-himachalene (18) [23] | Antioxidant, antiulcerogenic, anti-inflammatory, antitumor, and diuretic [24] | |
| Echinodorus macrophyllus (Kunth) Micheli | Leaves | Linalool (19), α-caryophyllene (20), β-caryophyllene (21) [25], isovitexin (22), and isoorientin (8) [26] | Diuretic, anti-inflammatory, treatment of kidney and liver disorders [27] | |
| Acmella oleracea (L.) R. K. Jansen | Flowers and leaves | Spilanthol (23), spermidine (24), spermine (25), and 3-acetylaleuritolic acid (26) [28][29][30] | Aphrodisiac, treatment of male sexual dysfunctions, diuretic, and anti-inflammatory [31][32] | |
| Rosmarinus officinalis L. | Leaves | Camphene (27), limonene (28), camphor (29), borneol (30), cineol (31), and linalool (19) [33] | Analgesic, anti-inflammatory, anticarcinogenic, antirheumatic, nephroprotective, spasmolytic, antihepatotoxic, atherosclerotic [34] | |
Harmine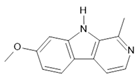 (1) |
Harmaline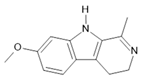 (2) |
Tetrahydroharmine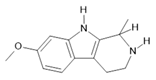 (3) |
Harmalinic acid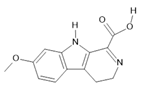 (4) |
Harmol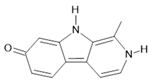 (5) |
Harmalol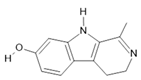 (6) |
Orientin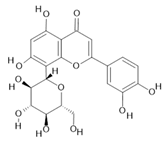 (7) |
Isoorientin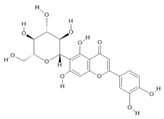 (8) |
Acetogenin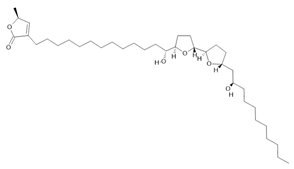 (9) |
|
δ-Cadinene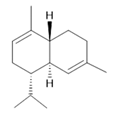 (10) |
α-Muurolene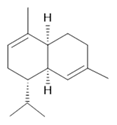 (11) |
Uncarine F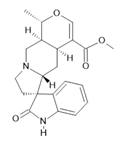 (12) |
Speciophylline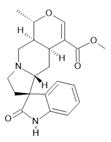 (13) |
Mitraphylline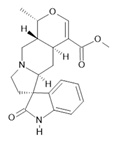 (14) |
Fisetin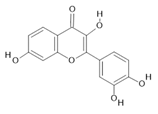 (15) |
Cyclosativene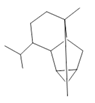 (16) |
Caryophyllene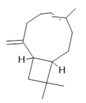 (17) |
α-himachalene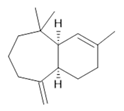 (18) |
Linalool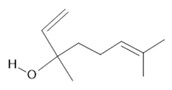 (19) |
α-caryophyllene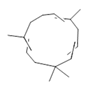 (20) |
β-caryophyllene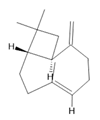 (21) |
Isovitexin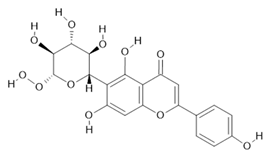 (22) |
Spilanthol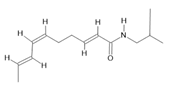 (23) |
|
Spermidine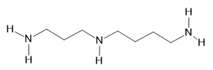 (24) |
Spermine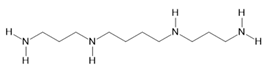 (25) |
3-acetylaleuritolic acid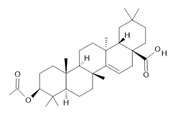 (26) |
Camphene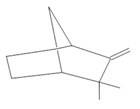 (27) |
|
Limonene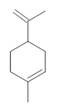 (28) |
Camphor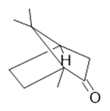 (29) |
Borneol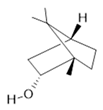 (30) |
Cineol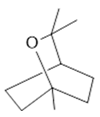 (31) |
|
The numbers in bold correspond to the molecular structures shown below.
2. Peganum harmala L.
The studied group belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Sapindales Juss. ex Bercht. & J. Presl, family Nitrariaceae Lindl., genus Peganum L. and species Peganum harmala L. [35]. The herbaceous P. harmala L. is perennial and branched, with leaves sectioned into three to five linear lobes. It produces whitish-yellow flowers and fruits in globular capsules with three chambers, containing black angular seeds [36]. It is commonly called wild rue, Syrian rue, or African rue [37].
Most species of the Nitrariaceae family contain alkaloids, which have been the subject of studies for their possible biological and pharmacological activity. For example, the studies by Niu et al. [38], when investigating the protective effect of harmine—the major compound isolated from P. harmala L.—in renal inflammation induced by lipopolysaccharide (LPS), as well as the respective molecular mechanisms involved, showed that pretreatment with harmine markedly alleviated the lesion kidney, reducing the release of renal biomarkers, inflammatory mediators, and the formation of malondialdehyde (MDA) and myeloperoxidase (MPO), while increasing superoxide dismutase (SOD) and glutathione (GSH) and reducing renal histopathological changes. Furthermore, in immunohistochemical staining and western blot analysis, the study indicated that the treatment with harmine suppressed the expression of the toll-like receptor 4 (TLR4), phosphorylation of nuclear factor kappa B (NF-κB) p65, and κBα inhibitor (IκBα), while the treatment also inhibited the expression of NLRP3, caspase-1, and interleukin-1β (IL-1β). In summary, pretreatment with harmine extracted from P. harmala L. can protect against LPS-induced acute kidney injury by attenuating oxidative stress and inflammatory responses and increasing antioxidant activity. The underlying mechanisms of harmine in mice with LPS-induced acute kidney injury may be related to the inhibition of the TLR4-NF-κB and NLRP3 pathways of the inflammasome.
Another study observed the effects of harmine on the renal activity of mice after cisplatin administration. The researchers demonstrated that there was a significant decrease in the total antioxidant capacity of the renal tissue, in the diameter of the renal corpuscles, and in the level of IL-10 expression in the group treated with cisplatin in relation to the control group, while the values of these parameters were significantly similar to those of the control group in the moderate or high dose groups treated with harmine + cisplatin. In addition, they noted significant increases in serum levels of urea and creatinine, Bowman’s space, amount of malondialdehyde, apoptosis rate, and gene expressions of TNF-α, NF-κB, IL-1β, and caspase-3 in the renal tissue of the cisplatin group compared to the control group, while these criteria did not differ in the harmine + cisplatin moderate or high dose groups. Thus, the study considered that harmine protected the kidneys against damage induced by cisplatin, and the antioxidant, anti-inflammatory, and anti-apoptotic properties of this compound were involved in the observed curative effect [14].
3. Passiflora edulis Sims
The species under study belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Malpighiales Juss. ex Bercht. & J. Presl, family Passifloraceae Juss. ex Roussel, genus Passiflora L. and species Passiflora edulis Sims, with wide ethnopharmacological use by the people of the Amazon. Passiflora edulis Sims is a vine, supported by axillary tendrils [39]. It consists of palmate leaves, usually three-lobed with serrated margins; large flowers, with long peduncles, whitish, with a purple and pink triple crown; fruits, oval-shaped berries, containing abundant flat ovoid seeds, covered by a yellowish or brownish aril [40]. It has a vast geographic distribution: Brazil, Paraguay, Argentina, Antilles (West Indies islands), Central America, Venezuela, and Ecuador. It is commonly called passion fruit [17].
Pharmacological trials have shown numerous activity from compounds obtained from P. edulis Sims, including anxiolytic, sedative, neuropathic pain [14], activity linked to alcoholism and narcotics use [41], anticonvulsant and anxiolytic activity [15], cognitive function and degenerative diseases [16], antioxidant, antitumor action, hypoglycemic action, obesity, and insomnia [17].
The species is rich in natural bioactive compounds, among them, a significant content of flavonoids. For example, orientin and isoorientin are compounds with potential hypoglycemic effects, pointed out in the study by Galdino et al. [13] when evaluating the therapeutic effect of the aqueous extract of the fruit peel of P. edulis Sims as an adjuvant to insulin, to confer nephroprotection against diabetes induced by streptozotocin in Wistar rats. In the study, those animals treated with P. edulis extract showed superior glycemic control, which resulted in a reduction in the urinary albumin/creatinine ratio; maintenance of basal levels of mRNA expression of Nphs1, Nphs2, and Wt1n in the renal tissue; expression of mRNA Lrp2; prevention of protein loss from the renal tissue to the urinary space; and maintenance of glomerular basement membrane thickness, hyalinization, and glomerular and tubulointerstitial fibrosis with values close to those of the control group and significantly lower than those in the diabetic group. Therefore, the extract of P. edulis revealed potential therapeutic action of nephroprotection due to the reduction and prevention of the development of diabetic kidney disease.
The protective effect of flavonoids from P. edulis Sims was evaluated in alloxan-induced diabetic Rattus norvegicus, in which researchers observed renal dysfunction in uncontrolled diabetic groups, given the increased production of free radicals, with probable cellular damage and tubular damage, resulting in renal inflammation. In the study, the biomarkers urea and creatinine were measured in the animals’ bloodstream. Diabetic animals that received the flavonoid fraction of P. edulis Sims had lower urea and creatinine values when compared to the control group [42].
4. Annona muricata L.
This plant species belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Magnoliales Bromhead, family Annonaceae Juss., genus Annona L. and species Annona muricata L. It has various uses in traditional indigenous medicine [35]. It is medium to large in size, reaching up to 10 m in height. Its leaves are green and shiny, with an oval shape and smooth texture, while its flowers are large and solitary, with thick, yellowish petals [43]. The species is widely distributed geographically, being found in several tropical regions of the world, including Central and South America, Africa, and Asia. It is known by several popular names, such as soursop, fruit of the count, and heart of queen, and is cultivated for its edible fruits, which have a sweet and slightly acidic taste [44].
In addition to its gastronomic uses, A. muricata L. is also consumed due to its medicinal properties. Many of these properties come from bioactive compounds present in its leaves, seeds, and fruits, with antioxidant, anti-inflammatory, antiparasitic, and anticancer activity [20].
A well-described example in the scientific literature is the species A. muricata L. This species has been studied for its bioactive metabolites, including acetogenins, and its constituents may have anticancer, hepatoprotective, neurotoxic, antinociceptive, antiulcerative, and chemopreventive activity [18].
A study of veterinary pharmacology and toxicology [45] demonstrated that A. muricata L. attenuates glycerol-induced nephrotoxicity in male albino rats through angiotensin-converting enzyme (ACE) signaling pathways. The methanolic extract of the leaves of A. muricata L., in that study, caused a significant decrease in the expression of the kidney injury molecule 1 (KIM-1) and exhibited antioxidant properties. This nephroprotective effect of the extract was observed by improving the levels of enzymatic and non-enzymatic antioxidants, suppressing inflammatory processes and inhibiting lipid peroxidation, thus revealing such antioxidant and anti-inflammatory properties.
5. Uncaria tomentosa (Willd.) DC.
The group studied has wide ethnopharmacological use in the Amazon. It belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Gentianales Juss. ex Bercht. & J. Presl, family Rubiaceae Juss., genus Uncaria Schreb. and species Uncaria tomentosa (Willd.) DC. [46]. Preliminary phytochemical screenings demonstrated the marked presence of tannins in species belonging to the Rubiaceae family. These plant species are widely disseminated in the culture of traditional people and communities, due to their richness in the production of bioactive compounds [47][48].
Other phytochemical studies have found tetracyclic and pentacyclic oxindole alkaloids, indole and β-carbonyl alkaloids, flavonoids [49], coumarins [50], proanthocyanidins, steroids, ursan-derived triterpenoids, and quinovic acid glycosides [21].
The species U. tomentosa (Willd.) DC. has been associated with several health benefits, such as antioxidant and immunomodulatory, anti-inflammatory, analgesic and anticancer action, in addition to other medicinal properties. In traditional medicine, the plant is used to treat a variety of conditions, including infections, arthritis, diabetes, gastrointestinal problems, and “kidney cleansing” [22].
The renal benefits of herbal medicines such as U. tomentosa (Willd.) DC. have been demonstrated in the studies by Vattimo and Silva [51], when performing a pretreatment with U. tomentosa (Willd.) DC. in experimental models of ischemia/reperfusion, in which there was functional protection assessed by increased creatinine clearance, reduced peroxidation, and urinary thiobarbituric acid reactive substances (TBARS), probably related to the antioxidant activity of the herbal medicine.
6. Hymenaea courbaril L.
The studied group belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Fabales Bromhead, family Fabaceae Lindl., genus Hymenaea L. and species Hymenaea courbaril L. [52]. The Fabaceae family is known to produce a wide variety of bioactive compounds, including tannins. The tannins present in Hymenaea courbaril L. have been the object of research for their potential biological effects, such as antioxidant, antiulcerogenic, anti-inflammatory, and antitumor properties [24]. Some studies also report the antiviral and antibacterial activity of these compounds [53].
Hymenaea courbaril L. has a wide distribution in South America and Central America; it is a large tree, reaching 15 to 20 m in height, and the trunk can be up to 1 m in diameter [54]. The flowers are pollinated by bats. Ripe fruits are eaten by rodents, birds, and monkeys, which, when breaking the fruits, release the seeds [55]. Its wood is considered valuable due to its high density and resistance to attack by xylophagous organisms. In the Amazon region, the species is known as jassaí, jataí, jataíba, jataíba stone, jataúba, jatel, jati, jatobá de anta, jutaí, jutaí açu, jutaí white, jutaí grande, and jutaí catinga [56].
The main phytochemical constituents found In the species are flavonoids, such as quercetin, kaempferol, and isorhamnetin, which are present in the leaves and fruits; tannins, such as catechins and proanthocyanidins, most commonly found in bark and seeds; fatty acids, including oleic and linoleic acid (seeds); and stilbenes, such as trans-resveratrol, found in the bark and fruit [57][58].
The tea produced from the bark of H. courbaril L. is indicated to treat kidney problems [59]. Pereira et al. [60] demonstrated the oxidizing activity of the methanolic fraction of H. courbaril L. seeds in mice treated with acetaminophen; the study showed the probable restoration of renal glutathione (GSH) levels in animals treated with the extract, in addition to reversing the increase in carbonylated proteins. Another study, using aqueous extracts of seed or bark of H. courbaril L., observed a reduction in renal levels of reactive substances to thiobarbituric acid 7 days after treatment [61].
7. Echinodorus macrophyllus (Kunth) Micheli
This species belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Alismatales R. Br. ex Bercht. & J. Presl, family Alismataceae Vent., genus Echinodorus Rich. and the species Echinodorus macrophyllus (Kunth) Micheli, widely used by traditional medicine in Brazil [62]. The Alismataceae family is known for the presence of several bioactive compounds. Many Alismataceae species have traditionally been used in folk medicine for the treatment of a vast range of diseases, due to their diuretic and anti-inflammatory effects, as well as in kidney and liver disorders [27]. Some of the bioactive compounds present in plants of this family, such as Echinodorus macrophyllus, have been the object of scientific investigation for their pharmacological properties and possible therapeutic applications [63].
Echinodorus macrophyllus is a perennial plant, herbaceous or subshrub, of aquatic origin, emerging from the water. It has rhizomes and can reach between 1 and 2 m in height. Its leaves are petiolate and oval, with a heart-shaped base and a sharp tip [64]. Popularly, the species is known as leather hat, water hyacinth, campanha tea, brejo tea, poor man’s tea, mineiro tea, congonha do brejo, brejo herb, and swamp herb [65].
According to Silva et al. [25], among the constituents produced by the species are the terpenic profile containing linalool, α- and β-caryophyllene, E-nerolidol, and phytol as predominant, as well as a variety of diterpenoids belonging to the same classes, such as the chapecoderines of the group labdanos. Furthermore, a (+)-3-carene derivative was detected, along with a significant proportion of carotenoids. Gasparotto et al. [26] demonstrated the presence of the flavonoids vitexin and isovitexin. While Garcia et al. [66] found the presence of phenylpropanoids in the species, such as ferulic and E-caffeoyl tartronic acid (2-E-caffeoyloxymalonic acid).
Traditionally, the Amazonian population uses extracts from the leaves of E. mac-rophyllus, from infusion, decoction, or maceration methods, in water or alcohol, to treat urinary system disorders, as they are known to be powerful diuretic agents. In view of popular usage, Nascimento et al. [67] demonstrated that preconditioning with E. macrophyllus attenuated cyclophosphamide-induced acute kidney injury in rats as evidenced by increased creatinine clearance and reduced oxidative metabolites in urine and increased reserve of antioxidant enzymes in renal tissue.
Studies carried out in a model of acute kidney injury induced by gentamicin found similar results of antioxidant protection of E. macrophyllus, when administering crude ethanolic extracts of leaves and fractions of E. macrophyllus by endogastric route, in normal rats or with acute tubular necrosis induced by gentamicin-cine. Thus, it was demonstrated that it produced a dose-dependent reduction in urine output. The extracts in question were effective in reversing all changes induced by gentamicin, such as polyuria and reduction in the glomerular filtration rate; in addition, the morphological changes induced by gentamicin were not observed in animals that were treated with extracts of E. macrophyllus concomitantly with gentamycin [63].
8. Acmella oleracea (L.) R. K. Jansen
The studied group belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Asterales Link, family Asteraceae Bercht. & J. Presl, genus Acmella Rich. ex Pers. and the species Acmella oleracea (L.) R. K. Jansen, which is widely used in medicine and cooking by traditional Amazonian people [68]. The Asteraceae family is known for the presence of bioactive compounds, such as sesquiterpene lactones and flavonoids [69]. Primarily for their medicinal properties, species in this family have traditionally been used to treat a wide range of ailments, including respiratory problems, inflammation, headaches, gastrointestinal problems, and infectious diseases [9]. Scientific research has focused on some of the bioactive compounds present in plants of this family, such as Acmella oleracea, in search of possible therapeutic applications, such as analgesic, anti-inflammatory, antimicrobial, and antioxidant activity [32].
Acmella oleracea is an important medicinal herb, which occurs in tropical and subtropical regions of the planet. It is an annual, perennial herbaceous, 30–40 cm high, semi-straight, or creeping, with cylindrical, a fleshy stem and decumbent branches, usually without roots at the nodes. The main root is pivotal, with abundant lateral branches. The leaves are opposite, membranous, and petiolate [70]. The species is popularly known as jambu, cress from pará, abecedária, cress bravo, cress from Brazil, cress from the north, buttercup, crazy herb, jabuaçu, and nhambu [32].
Acmella oleracea is used in northern Brazil for the treatment of various diseases, such as tuberculosis, flu, cough, and rheumatism, and as an anti-inflammatory; in addition, hydroethanolic formulations with this species are popularly used as a female aphrodisiac, for treatment of male sexual dysfunctions, and as a diuretic [31][32].
Regarding the production of metabolites, A. oleracea is a rich source of secondary metabolites, and its phytochemistry has been widely investigated [71]. Borges et al. [72] observed an increase of 31.6% in the content of spilanthol and 16.8% of flavonoids in the inflorescences and higher contents of total phenols, carotenoids, spermidine, and spermine in the leaves and flowers of jambú. The work by Abeysiri et al. [28] revealed that alkaloids, flavonoids, saponins, steroid glycosides, and tannins are distributed in all parts of the plant. Going into more detail about the phytochemical composition of A. oleracea, several triterpenoids were found, such as 3-acetylaleuritolic acid, β-sitostenone, and stigmasterol. Furthermore, steroidal glycosides, namely, stigmasteryl-3-O-β-D-glucopyranoside and β-sitosteryl-3-O-β-D-glucopyranoside, have been identified. Several phenolic compounds were also detected, such as vanillic, trans-ferulic, and trans-isoferulic acids; scopoletin; and fatty acids such as n-hexadecanoic and n-tetradecanoic acids [28][29][30].
Some studies have observed a marked diuretic action of aqueous extract of A. oleracea inflorescences in rats; the authors have described an increase in Na+ and K+ levels and a reduction in osmolarity in the urine of animals treated with the extract [73]. Yadav and collaborators [74] showed that the ethanolic extract of A. oleracea in rats provided diuresis similar to that produced by the action of furosemide. Gerbino et al. [75] consider that the inhibition of cyclic AMP induced by spilanthol negatively modulates the mechanisms of urine concentration. Furthermore, the mechanisms of action on the kidney show that A. oleracea is a promising source of compounds with diuretic activity.
9. Rosmarinus officinalis L.
The species belongs to the kingdom Plantae, class Equisetopsida C. Agardh, order Lamiales Bromhead, family Lamiaceae Martinov, genus Rosmarinus L. and species Rosma-rinus officinalis L.; it has wide ethnopharmacological use [76]. The Lamiaceae family is one of the most important herbaceous families; it is composed of an immense variety of plant species with biological and medicinal applications [34]. This family includes numerous aromatic spices, including Rosmarinus officinalis L., a plant species commonly known as rosemary, which is useful in cooking due to its characteristic aroma; it is widely used by indigenous populations where it grows spontaneously [77].
Rosmarinus officinalis is a shrubby herb, widely used in culinary, medicinal, and commercial applications, including the fragrance and food industries [78]. The leaves (fresh or dried) are consumed due to the characteristic odor that they offer to the dish. They are also consumed in small amounts in the form of tea, while extracts of R. officinalis are regularly used for their active natural antioxidant properties to improve shelf life of perishable foods [79].
Phytochemical screenings carried out on the species revealed 0.5% to 2.5% volatile oil in the leaves. Among the phytocompounds, the species exhibits the presence of monoterpene hydrocarbons (alpha and beta-pinene), camphene, limonene, camphor (10% to 20%), borneol, cineol, linalool, and verbinol [33]. In addition to numerous volatile and aromatic components, the species has flavonoids, such as diosmetin, diosmin, genkwanin, luteolin, hispidulin, and apigenin, as well as terpenoid compounds such as triterpenes (oleanolic and ursolic acid) and diterpene carnosol. Among the phenols found in the species are caffeic, chlorogenic, labiatic, neochlorogenic, and rosmarinic acids, as well as a considerable number of salicylates [78][79][80].
Among the ethnomedicinal applications for R. officinalis are analgesic, anti-inflammatory, anticarcinogenic, antirheumatic, nephroprotective, spasmolytic, antihepatotoxic, atherosclerotic, carminative, and choleretic action. It also offers protection against UV and gamma radiation and improvement of stress [34].
Zohrabi et al. [81] investigated the effect of an oral extract of R. officinalis on acute renal failure (ARF) disorders induced by ischemia/reperfusion in rats. The authors showed that the aqueous extract of R. officinalis suffered the oxidative stress marker malondialdehyde (MDA), increased the ferric antioxidant reducing power (FRAP) compared to the vehicle groups and, regarding the histopathological analyses, observed a significant reduction in vessel management, disturbance of the tubules, and Bowman’s Capsule space compared to the vehicle groups.
Another study evaluated the effectiveness of R. officinalis essential oil (REO) against changes induced by potassium dichromate in the kidneys of male rats, in which they injected hexavalent chromium to induce renal dysfunction (oxidative damage and alterations in the antioxidant defense system, and histopathological and immunohistochemical alterations). The animals were treated with REO before or after the induction of renal dysfunction, resulting in an improvement in the toxic effect by extinguishing, chelating, and detoxifying free radicals and enhancing the state of antioxidant defense [82].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28176411
References
- Morton, C.V. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Schwarz, M.J.; Houghton, P.J.; Rose, S.; Jenner, P.; Lees, A.D. Activities of Extract and Constituents of Banisteriopsis Caapi Relevant to Parkinsonism. Pharmacol. Biochem. Behav. 2003, 75, 627–633.
- Pic-Taylor, A.; da Motta, L.G.; de Morais, J.A.; Junior, W.M.; Santos, A.d.F.A.; Campos, L.A.; Mortari, M.R.; von Zuben, M.V.; Caldas, E.D. Behavioural and Neurotoxic Effects of Ayahuasca Infusion (Banisteriopsis Caapi and Psychotria Viridis) in Female Wistar Rat. Behav. Process. 2015, 118, 102–110.
- Samoylenko, V.; Rahman, M.d.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.-H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis Caapi, a Unique Combination of MAO Inhibitory and Antioxidative Constituents for the Activities Relevant to Neurodegenerative Disorders and Parkinson’s Disease. J. Ethnopharmacol. 2010, 127, 357–367.
- Salahshoor, M.; Roshankhah, S.; Motavalian, V.; Jalili, C. Effect of Harmine on Nicotine-Induced Kidney Dysfunction in Male Mice. Int. J. Prev. Med. 2019, 10, 97.
- Santos, B.W.L.; Moreira, D.C.; Borges, T.K.d.S.; Caldas, E.D. Components of Banisteriopsis Caapi, a Plant Used in the Preparation of the Psychoactive Ayahuasca, Induce Anti-Inflammatory Effects in Microglial Cells. Molecules 2022, 27, 2500.
- Sawant, M.; Isaac, J.C.; Narayanan, S. Analgesic Studies on Total Alkaloids and Alcohol Extracts of Eclipta Alba (Linn.) Hassk. Phytother. Res. 2004, 18, 111–113.
- Morales-García, J.A.; de la Fuente Revenga, M.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The Alkaloids of Banisteriopsis Caapi, the Plant Source of the Amazonian Hallucinogen Ayahuasca, Stimulate Adult Neurogenesis in Vitro. Sci. Rep. 2017, 7, 5309.
- Batista, L.L.; do Nascimento, L.C.; Guimarães, G.F.; Matias Pereira, A.C.; Koga, R.d.C.R.; Teixeira dos Santos, A.V.T.d.L.; Fernandes, C.P.; Teixeira, T.A.; Hu, Y.; Hu, X.; et al. A Review of Medicinal Plants Traditionally Used to Treat Male Sexual Dysfunctions—The Overlooked Potential of Acmella oleracea (L.) R.K. Jansen. Pharmacogn. Rev. 2021, 15, 1–11.
- Rasouli, H.; Yarani, R.; Pociot, F.; Popović-Djordjević, J. Anti-Diabetic Potential of Plant Alkaloids: Revisiting Current Findings and Future Perspectives. Pharmacol. Res. 2020, 155, 104723.
- Zenk, M.H.; Juenger, M. Evolution and Current Status of the Phytochemistry of Nitrogenous Compounds. Phytochemistry 2007, 68, 2757–2772.
- Ghanbari, A.; Jalili, C.; Salahshoor, M.; Javanmardy, S.; Ravankhah, S.; Akhshi, N. Harmine Mitigates Cisplatin-Induced Renal Injury in Male Mice through Antioxidant, Anti-Inflammatory, and Anti-Apoptosis Effects. Res. Pharm. Sci. 2022, 17, 417.
- Araújo Galdino, O.; de Souza Gomes, I.; Ferreira de Almeida Júnior, R.; Conceição Ferreira de Carvalho, M.I.; Abreu, B.J.; Abbott Galvão Ururahy, M.; Cabral, B.; Zucolotto Langassner, S.M.; Costa de Souza, K.S.; Augusto de Rezende, A. The Nephroprotective Action of Passiflora edulis in Streptozotocin-Induced Diabetes. Sci. Rep. 2022, 12, 17546.
- Deng, J.; Zhou, Y.; Bai, M.; Li, H.; Li, L. Anxiolytic and Sedative Activities of Passiflora edulis f. Flavicarpa. J. Ethnopharmacol. 2010, 128, 148–153.
- Sena, L.M.; Zucolotto, S.M.; Reginatto, F.H.; Schenkel, E.P.; De Lima, T.C.M. Neuropharmacological Activity of the Pericarp of Passiflora edulis Flavicarpa Degener: Putative Involvement of C -Glycosylflavonoids. Exp. Biol. Med. 2009, 234, 967–975.
- Doungue, H.T.; Kengne, A.P.N.; Kuate, D. Neuroprotective Effect and Antioxidant Activity of Passiflora edulis Fruit Flavonoid Fraction, Aqueous Extract, and Juice in Aluminum Chloride-Induced Alzheimer’s Disease Rats. Nutrire 2018, 43, 23.
- Taïwe, G.S.; Kuete, V. Passiflora edulis. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 513–526.
- Chan, W.-J.J.; McLachlan, A.J.; Hanrahan, J.R.; Harnett, J.E. The Safety and Tolerability of Annona muricata Leaf Extract: A Systematic Review. J. Pharm. Pharmacol. 2019, 72, 1–16.
- Gyesi, J.N.; Opoku, R.; Borquaye, L.S. Chemical Composition, Total Phenolic Content, and Antioxidant Activities of the Essential Oils of the Leaves and Fruit Pulp of Annona muricata L. (Soursop) from Ghana. Biochem. Res. Int. 2019, 2019, 4164576.
- Adewole, S.; Caxton-Martins, E. Morphological Changes and Hypoglycemic Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Pancreatic β-Cells of Streptozotocin-Treated Diabetic Rats. Afr. J. Biomed. Res. 2009, 9.
- Pilarski, R.; Zieliński, H.; Ciesiołka, D.; Gulewicz, K. Antioxidant Activity of Ethanolic and Aqueous Extracts of Uncaria tomentosa (Willd.) DC. J. Ethnopharmacol. 2006, 104, 18–23.
- Batiha, G.E.-S.; Magdy Beshbishy, A.; Wasef, L.; Elewa, Y.H.A.; Abd El-Hack, M.E.; Taha, A.E.; Al-Sagheer, A.A.; Devkota, H.P.; Tufarelli, V. Uncaria tomentosa (Willd. Ex Schult.) DC.: A Review on Chemical Constituents and Biological Activities. Appl. Sci. 2020, 10, 2668.
- Khoo, S.F.; Oehlschlager, A.C.; Ourisson, G. Structure and Stereochemistry of the Diterpenes of Hymenaea courbaril (Caesalpinioideae) Seed Pod Resin. Tetrahedron 1973, 29, 3379–3388.
- Spera, K.D.; Figueiredo, P.A.; Santos, P.C.; Barbosa, F.C.; Alves, C.P.; Dokkedal, A.L.; Saldanha, L.L.; Silva, L.P.; Figueiredo, C.R.; Ferreira, P.C.; et al. Genotoxicity, Anti-Melanoma and Antioxidant Activities of Hymenaea courbaril L. Seed Extract. An. Acad. Bras. Cienc. 2019, 91, e20180446.
- Silva, T.M.; Miranda, R.R.S.; Ferraz, V.P.; Pereira, M.T.; de Siqueira, E.P.; Alcântara, A.F.C. Changes in the Essential Oil Composition of Leaves of Echinodorus macrophyllus Exposed to γ-Radiation. Rev. Bras. Farmacogn. 2013, 23, 600–607.
- Gasparotto, F.; Lívero, F.; Palozi, R.; Ames, M.; Nunes, B.; Donadel, G.; Ribeiro, R.; Lourenço, E.; Kassuya, C.; Junior, A. Heart-Protective Effects of Echinodorus grandiflorus in Rabbits That Are Fed a High-Cholesterol Diet. Planta Med. 2018, 84, 1271–1279.
- Fernandes, D.C.; Martins, B.P.M.P.; Medeiros, D.L.; Santos, S.V.; Gayer, C.R.; Velozo, L.S.; Coelho, M.G. Antinociceptive and Anti-Inflammatory Activities of the Hexanic Extract of “Echinodorus macrophyllus” (Kunth) Micheli in Mice. Braz. J. Health Biomed. Sci. 2019, 18, 25–32.
- Spinozzi, E.; Ferrati, M.; Baldassarri, C.; Cappellacci, L.; Marmugi, M.; Caselli, A.; Benelli, G.; Maggi, F.; Petrelli, R. A Review of the Chemistry and Biological Activities of Acmella oleracea (“Jambù”, Asteraceae), with a View to the Development of Bioinsecticides and Acaricides. Plants 2022, 11, 2721.
- Abeysiri, G.R.P.I.; Dharmadasa, R.M.; Abeysinghe, D.C.; Samarasinghe, K. Screening of Phytochemical, Physico-Chemical and Bioactivity of Different Parts of Acmella oleraceae Murr. (Asteraceae), a Natural Remedy for Toothache. Ind. Crops Prod. 2013, 50, 852–856.
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive Metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867.
- Batista, L.L.; Koga, R.d.C.R.; Teixeira, A.V.T.d.L.; Teixeira, T.A.; de Melo, E.L.; Carvalho, J.C.T. Clinical Safety of a Pharmaceutical Formulation Containing an Extract of Acmella oleracea (L.) in Patients With Premature Ejaculation: A Pilot Study. Am. J. Men’s Health 2023, 17, 155798832311678.
- Souza, G.; Dias Ribeiro da Silva, I.; Duarte Viana, M.; Costa de Melo, N.; Sánchez-Ortiz, B.; Maia Rebelo de Oliveira, M.; Ramos Barbosa, W.; Maciel Ferreira, I.; Tavares Carvalho, J. Acute Toxicity of the Hydroethanolic Extract of the Flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies. Pharmaceuticals 2019, 12, 173.
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis Essential Oil: A Review of Its Phytochemistry, Anti-Inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45.
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543.
- Linnaeus, C.V. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Improving Health Benefits with Considering Traditional and Modern Health Benefits of Peganum Harmala. Clin. Phytosci. 2021, 7, 18.
- Niroumand, M.C.; Farzaei, M.H.; Amin, G. Medicinal Properties of Peganum Harmala L. in Traditional Iranian Medicine and Modern Phytotherapy: A Review. J. Tradit. Chin. Med. 2015, 35, 104–109.
- Niu, X.; Yao, Q.; Li, W.; Zang, L.; Li, W.; Zhao, J.; Liu, F.; Zhi, W. Harmine Mitigates LPS-Induced Acute Kidney Injury through Inhibition of the TLR4-NF-ΚB/NLRP3 Inflammasome Signalling Pathway in Mice. Eur. J. Pharmacol. 2019, 849, 160–169.
- Sims, J. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- De Melo, N.F.; Cervi, A.C.; Guerra, M. Karyology and Cytotaxonomy of the Genus Passiflora L. (Passifloraceae). Plant Syst. Evol. 2001, 226, 69–84.
- Zhang, Y.-J.; Zhou, T.; Wang, F.; Zhou, Y.; Li, Y.; Zhang, J.-J.; Zheng, J.; Xu, D.-P.; Li, H.-B. The Effects of Syzygium samarangense, Passiflora edulis and Solanum muricatum on Alcohol-Induced Liver Injury. Int. J. Mol. Sci. 2016, 17, 1616.
- Salles, B.C.C.; Leme, K.C.; da Silva, M.A.; da Rocha, C.Q.; Tangerina, M.M.P.; Vilegas, W.; Figueiredo, S.A.; da Silveria Duarte, S.M.; Rodrigues, M.R.; de Araújo Paula, F.B. Protective Effect of Flavonoids from Passiflora edulis Sims on Diabetic Complications in Rats. J. Pharm. Pharmacol. 2021, 73, 1361–1368.
- Leboeuf, M.; Cavé, A.; Bhaumik, P.K.; Mukherjee, B.; Mukherjee, R. The Phytochemistry of the Annonaceae. Phytochemistry 1980, 21, 2783–2813.
- Moghadamtousi, S.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.; Kadir, H. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658.
- Adedapo, A.A.; Oni, O.A.; Falayi, O.O.; Ogunmiluyi, I.O.; Ogunpolu, B.S.; Omobowale, T.O.; Oyagbemi, A.A.; Oguntibeju, O.O.; Yakubu, M.A. Annona muricata Mitigates Glycerol-Induced Nephrotoxicities in Male Albino Rats through Signaling Pathways of Angiotensin Conversion Enzyme, Kidney Injury Molecule-1, and Antioxidant Properties. Sci. Afr. 2022, 16, e01225.
- Bremekamp, C.E.B. Tropicos.Org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Conserva, L.; Ferreira, J. Borreria and Spermacoce Species (Rubiaceae): A Review of Their Ethnomedicinal Properties, Chemical Constituents, and Biological Activities. Pharmacogn. Rev. 2012, 6, 46.
- Kala, S.C. Medicinal Attributes of Family Rubiaceae. Int. J. Pharm. Biol. Sci. 2015, 5, 179–181.
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, Phytochemistry and Pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29.
- Valente, L.M.M.; Bizarri, C.H.B.; Liechocki, S.; Barboza, R.S.; da Paixão, D.; Almeida, M.B.S.; Benevides, P.J.C.; Magalhães, A.; Siani, A.C. Kaempferitrin from Uncaria Guianensis (Rubiaceae) and Its Potential as a Chemical Marker for the Species. J. Braz. Chem. Soc. 2009, 20, 1041–1045.
- Machado, D.I.; de Oliveira Silva, E.; Ventura, S.; Vattimo, M.d.F.F. The Effect of Curcumin on Renal Ischemia/Reperfusion Injury in Diabetic Rats. Nutrients 2022, 14, 2798.
- Mostacedo, C.B.; Uslar, Y. Plantas Silvestres Con Frutos y Semillas Comestibles Del Departamento de Santa Cruz, Bolivia: Un Inventario Preliminar. Rev. Soc. Boliv. Botánica 1999, 2, 203–226.
- Sales, G.W.P.; Batista, A.H.M.; Rocha, L.Q.; Nogueira, N.A.P. Efeito Antimicrobiano e Modulador Do Óleo Essencial Extraído Da Casca de Frutos Da Hymenaea courbaril L. Rev. Ciências Farm. Básica E Apl. 2014, 35, 709–715.
- Tiago, P.V.; Larocca, D.; da Silva, I.V.; Carpejani, A.A.; Tiago, A.V.; Dardengo, J.d.F.E.; Rossi, A.A.B. Caracterização Morfoanatômica, Fitoquímica e Histoquímica de Hymenaea courbaril (Leguminosae), Ocorrente Na Amazônia meridional. Rodriguésia 2020, 71, e02182018.
- Duarte, M.M.; de Paula, S.R.P.; Ferreira, F.R.d.L.; Nogueira, A.C. Morphological Characterization of Fruit, Seed and Seedling and Germination of Hymenaea courbaril L. (Fabaceae) (‘Jatobá’). J. Seed Sci. 2016, 38, 204–211.
- Gorchov, D.L.; Palmeirim, J.M.; Ascorra, C.F. Dispersal of Seeds of Hymenaea courbaril (Fabaceae) in a Logged Rain Forest in the Peruvian Amazonian. Acta Amazon. 2004, 34, 251–259.
- Dos Santos, A.G.; Sivieri, K.; Miglioli da Mata, B.P.; Salgaço, M.K.; Silva do Sacramento, L.V. Jatobá (Hymenaea courbaril L.). In Handbook of Phytonutrients in Indigenous Fruits and Vegetables; CABI: Wallingford, UK, 2022; pp. 266–280.
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142.
- Campelo, D.S.; Campelo, T.P.T.; Ferraz, A.B.F. Avaliação das Características Químicas e Biológicas da Garrafada de Carobinha; Digital Editora: Canoas, Brazil, 2021.
- Gindri-Sinhorin, V. Avaliação Antioxidante Do Extrato Da Semente de Hymenaea courbaril l. (Jatobá) Em Camundongos Tratados Com Acetaminofeno. Rev. Cuba. Plantas Med. 2020, 25, 1–13.
- de Jesus Lisboa, E.M.; Albiero, L.R.; Melchiors, N.; de Paula Borges, W.S.; da Silva Lima, V.; Rodrigues, F.D.; Sinhorin, V.D.G.; Castoldi, L. Evaluation of the Antitumor and Antioxidant Effects of Jatobá (Hymenaea courbaril) Extracts/Avaliação Do Efeito Antitumoral e Antioxidante de Extratos Do Jatobá (Hymenaea courbaril). Braz. J. Dev. 2021, 7, 116001–116018.
- Micheli, M. Tropicos.org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Portella, V.G.; Cosenza, G.P.; Diniz, L.R.L.; Pacheco, L.F.; Cassali, G.D.; Caliari, M.V.; Brandão, M.d.G.L.; Vieira, M.A.R. Nephroprotective Effect of Echinodorus macrophyllus Micheli on Gentamicin-Induced Nephrotoxicity in Rats. Nephron Extra 2012, 2, 177–183.
- Dutra, R.C.; Tavares, C.Z.; Ferraz, S.O.; Sousa, O.V.; Pimenta, D.S. Investigação Das Atividades Analgésica e Antiinflamatória Do Extrato Metanólico Dos Rizomas de Echinodorus grandiflorus. Rev. Bras. Farmacogn. 2006, 16, 469–474.
- Marques, A.M.; Provance, D.W.; Kaplan, M.A.C.; Figueiredo, M.R. Echinodorus grandiflorus: Ethnobotanical, Phytochemical and Pharmacological Overview of a Medicinal Plant Used in Brazil. Food Chem. Toxicol. 2017, 109, 1032–1047.
- De Fafia Garcia, E.; de Oliveira, M.A.; Godin, A.M.; Ferreira, W.C.; Bastos, L.F.S.; de Matos Coelho, M.; Braga, F.C. Antiedematogenic Activity and Phytochemical Composition of Preparations from Echinodorus grandiflorus Leaves. Phytomedicine 2010, 18, 80–86.
- Do Nascimento, E.L.; Watanabe, M.; da Fonseca, C.D.; Schlottfeldt, F.d.S.; Vattimo, M.d.F.F. Renoprotective Effect of the Echinodorus macrophyllus in Induced Renal Injury. Acta Paul. Enferm. 2014, 27, 12–17.
- Jansen, R.K.; Tropicos.org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 14 June 2023).
- Kostić, A.Ž.; Janaćković, P.; Kolašinac, S.M.; Dajić Stevanović, Z.P. Balkans’ Asteraceae Species as a Source of Biologically Active Compounds for the Pharmaceutical and Food Industry. Chem. Biodivers. 2020, 17, e2000097.
- De Oliveira, P.R.; Anholeto, L.; Ferreira Rodrigues, R.; Arnosti, A.; Bechara, G.; de Carvalho Castro, K.; Camargo-Mathias, M. Cytotoxic Effects of Extract of Acmella oleracea in the Ovaries and Midgut of Rhipicephalus sanguineus Latreille, 1806 (Acari: Ixodidae) Female Ticks. J. Microsc. Ultrastruct. 2019, 7, 28.
- Borges, L.d.S.; Vieir, M.A.; Marqu, M.O.; Vianello, F.; Lima, G.P. Influence of Organic and Mineral Soil Fertilization on Essential Oil of Spilanthes Oleracea Cv. Jambuarana. Am. J. Plant Physiol. 2012, 7, 135–142.
- Da Silva Borges, L.; de Souza Vieira, M.C.; Vianello, F.; Goto, R.; Lima, G.P.P. Antioxidant Compounds of Organically and Conventionally Fertilized Jambu (Acmella oleracea). Biol. Agric. Hortic. 2016, 32, 149–158.
- Ratnasooriya, W.D.; Pieris, K.P.P.; Samaratunga, U.; Jayakody, J.R.A.C. Diuretic Activity of Spilanthes acmella Flowers in Rats. J. Ethnopharmacol. 2004, 91, 317–320.
- Yadav, R.; Kharya, M.D.; Yadav, N.; Savadi, R. Diuretic Activity of Spilanthes acmella Murr. Leaves Extract in Rats. Int. J. Res. Pharm. Chem. 2011, 1, 57–61.
- Gerbino, A.; Schena, G.; Milano, S.; Milella, L.; Barbosa, A.F.; Armentano, F.; Procino, G.; Svelto, M.; Carmosino, M. Spilanthol from Acmella oleracea Lowers the Intracellular Levels of CAMP Impairing NKCC2 Phosphorylation and Water Channel AQP2 Membrane Expression in Mouse Kidney. PLoS ONE 2016, 11, e0156021.
- Zappi, D.C.; Filardi, F.L.R.; Leitman, P.; Souza, V.C.; Walter, B.M.T.; Pirani, J.R.; Morim, M.P.; Queiroz, L.P.; Cavalcanti, T.B.; Mansano, V.F.; et al. Growing Knowledge: An Overview of Seed Plant Diversity in Brazil. Rodriguésia 2015, 66, 1085–1113.
- Fernandez, L.; Duque, S.; Sanchez, I.; Quiñones, D.; Rodriguez, F.; Garcia-Abujeta, J.L. Allergic Contact Dermatitis from Rosemary (Rosmarinus officinalis L.). Contact Dermat. 1997, 37, 248–249.
- Emami, F.; Ali-Beig, H.; Farahbakhs, S.; Mojabi, N.; Rastegar-M, B.; Arbabian, S.; Kazemi, M.; Tekieh, E.; Golmanesh, L.; Ranjbaran, M.; et al. Hydroalcoholic Extract of Rosemary (Rosmarinus officinalis L.) and Its Constituent Carnosol Inhibit Formalin-Induced Pain and Inflammation in Mice. Pak. J. Biol. Sci. 2013, 16, 309–316.
- Sotelo-Félix, J.I.; Martinez-Fong, D.; Muriel, P.; Santillán, R.L.; Castillo, D.; Yahuaca, P. Evaluation of the Effectiveness of Rosmarinus officinalis (Lamiaceae) in the Alleviation of Carbon Tetrachloride-Induced Acute Hepatotoxicity in the Rat. J. Ethnopharmacol. 2002, 81, 145–154.
- Almela, L.; Sánchez-Muñoz, B.; Fernández-López, J.A.; Roca, M.J.; Rabe, V. Liquid Chromatograpic–Mass Spectrometric Analysis of Phenolics and Free Radical Scavenging Activity of Rosemary Extract from Different Raw Material. J. Chromatogr. A 2006, 1120, 221–229.
- Zohrabi, M. The Study of 24 h Post Treatment Effects of the Aqueous Extract of Rosmarinus officinalis after Renal Ischemia/Reperfusion in Rat. J. Physiol. Pathophysiol. 2012, 3, 12–19.
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Rosmarinus officinalis Essential Oil Modulates Renal Toxicity and Oxidative Stress Induced by Potassium Dichromate in Rats. J. Trace Elem. Med. Biol. 2021, 67, 126791.
This entry is offline, you can click here to edit this entry!
