Phenolic compounds present in grapes and their derivatives, such as grape juices, represent today a broad area of research, given the benefits they have on human health. Grape juice can be produced from any grape variety once it matures appropriately. However, only in traditional wine-producing regions are grape juices produced from Vitis vinifera grape varieties. For example, Brazilian grape juices are essentially made from Vitis labrusca grape varieties, known as American or hybrid, as they preserve their characteristics, such as the natural flavor after pasteurization. Grapes are one of the richest sources of phenolic compounds among fruits. Therefore, grape juices have been broadly studied due to their composition in phenolic compounds and their potential beneficial effects on human health, specifically the ability to prevent various diseases associated with oxidative stress, including cancers and cardiovascular and neurodegenerative diseases.
- grape juice
- phenolic compounds
- antioxidant activity
- bioactive compounds
- sensory analysis
1. General Introduction
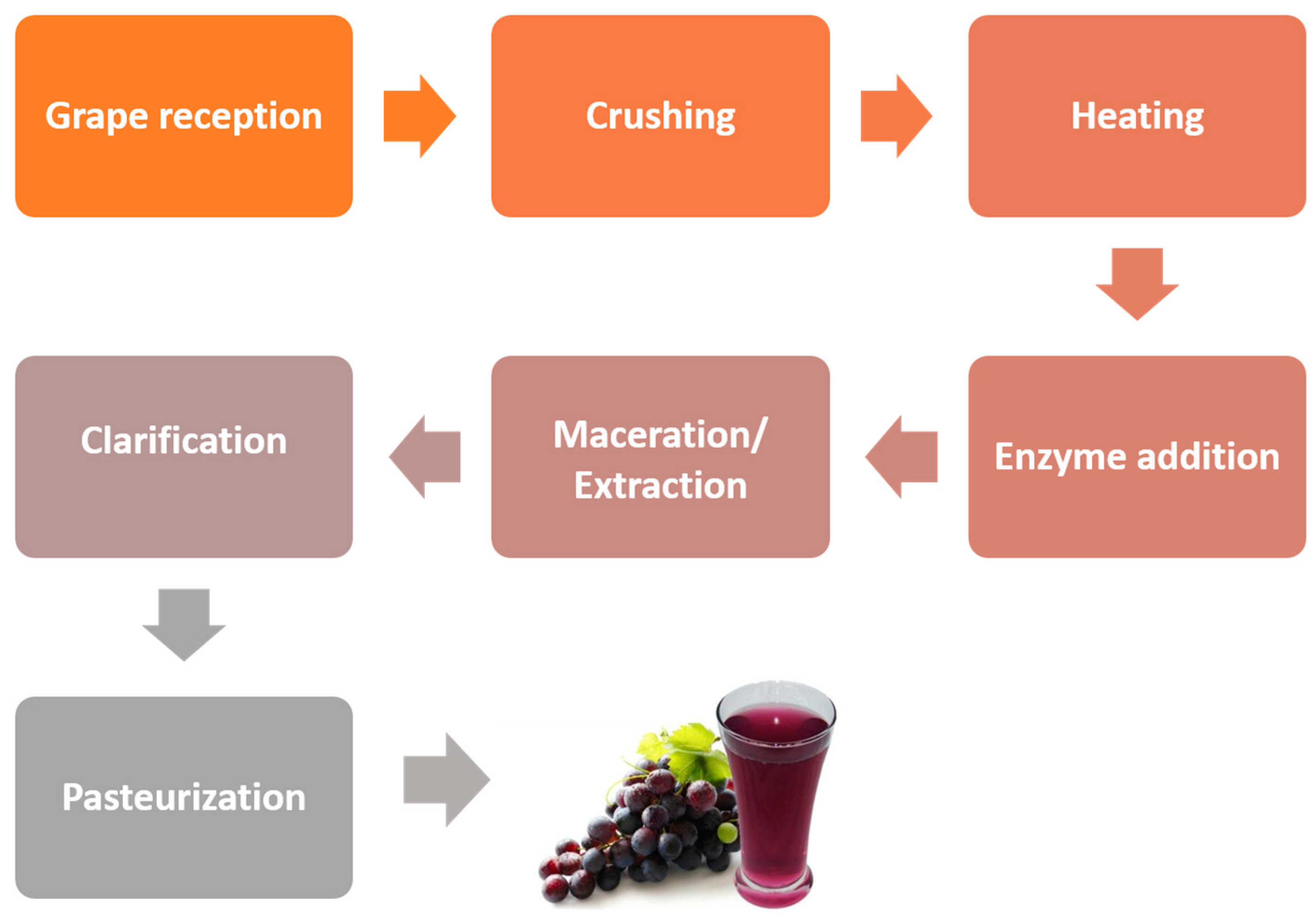
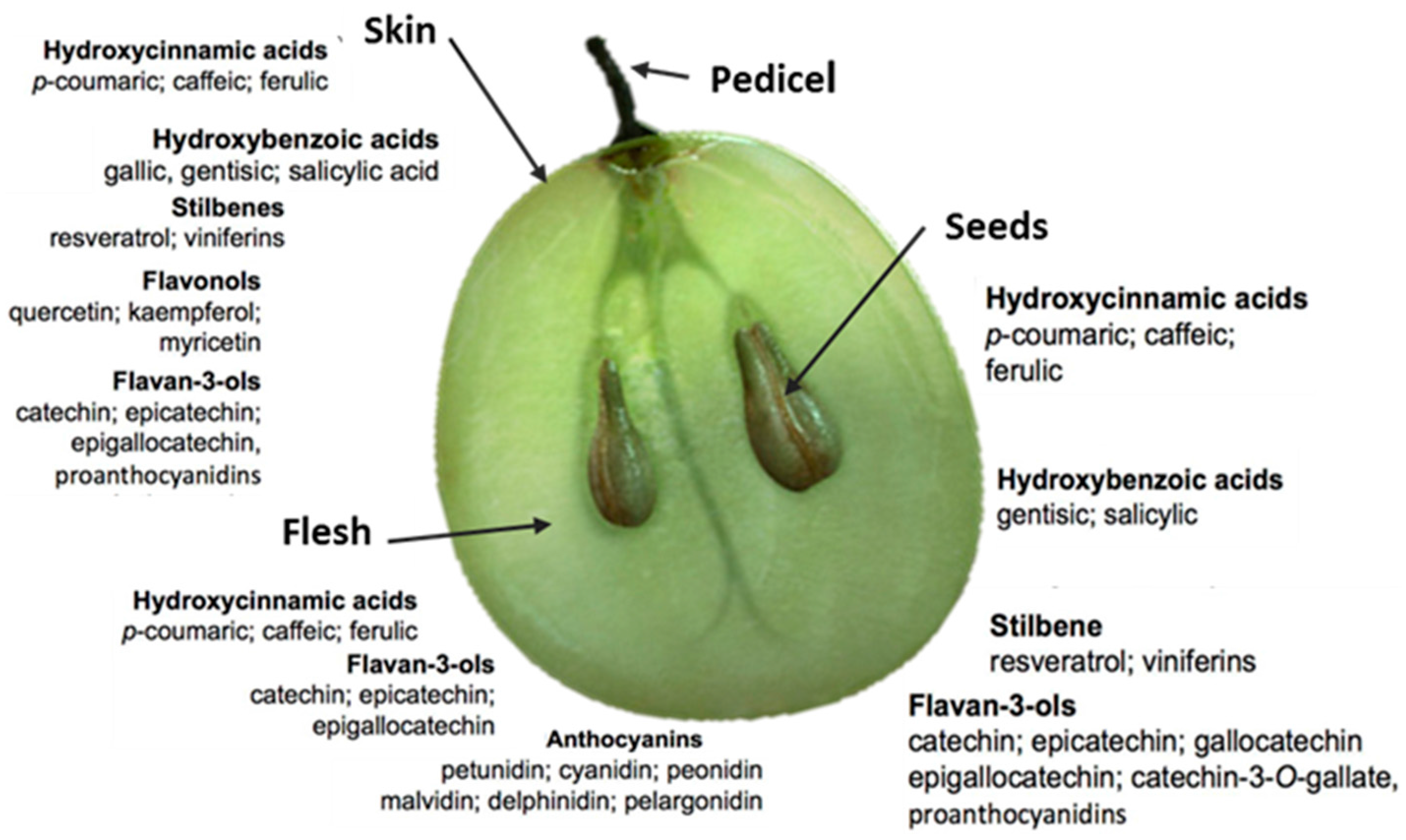
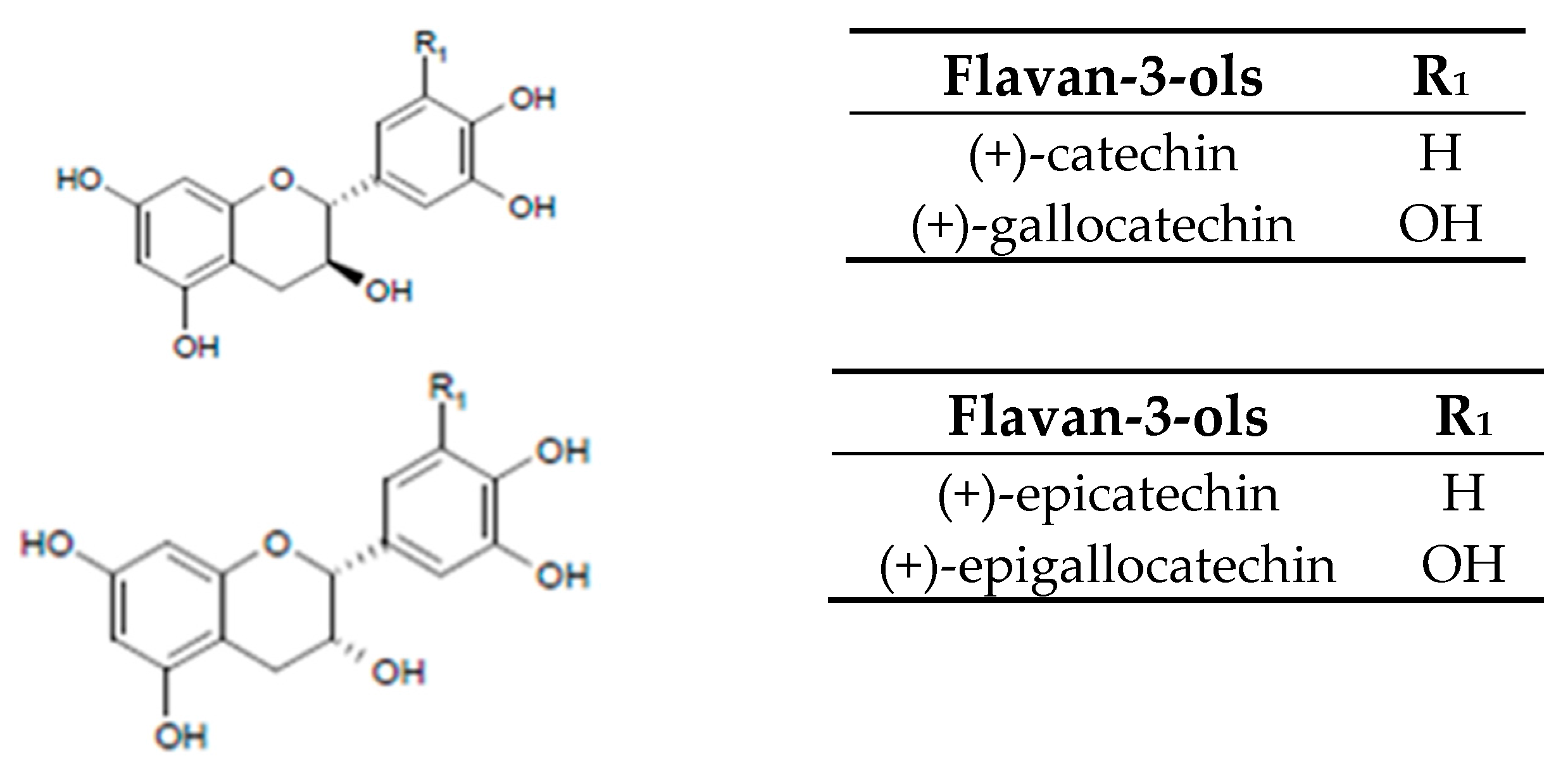
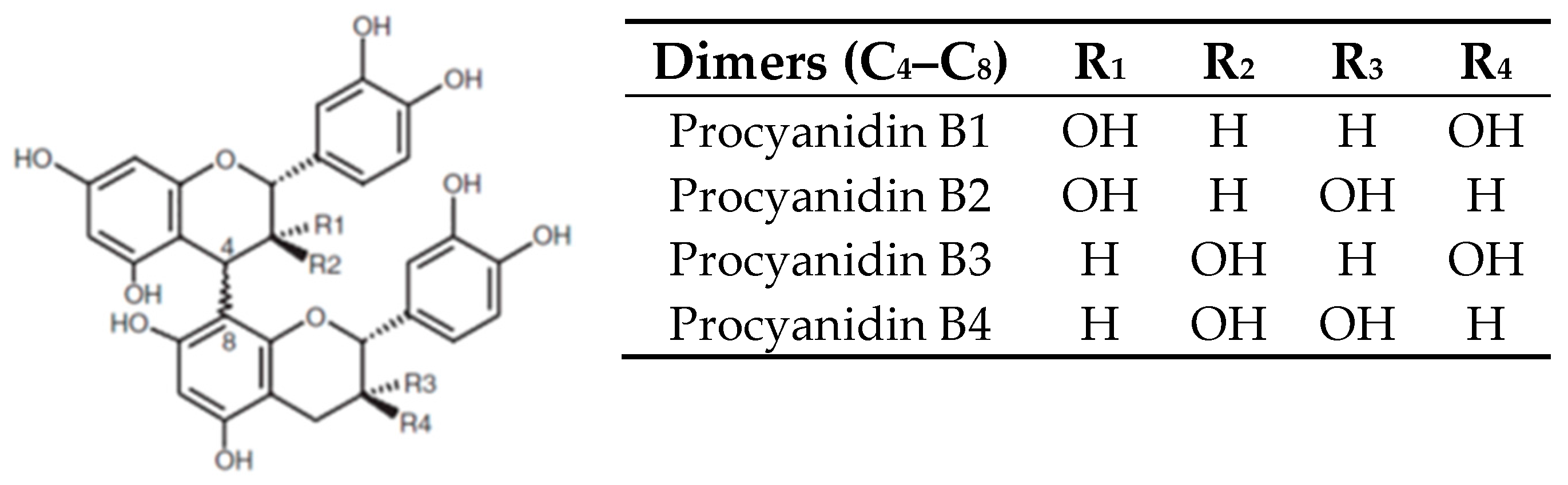
2. Grape Juice Production and Phenolic Composition
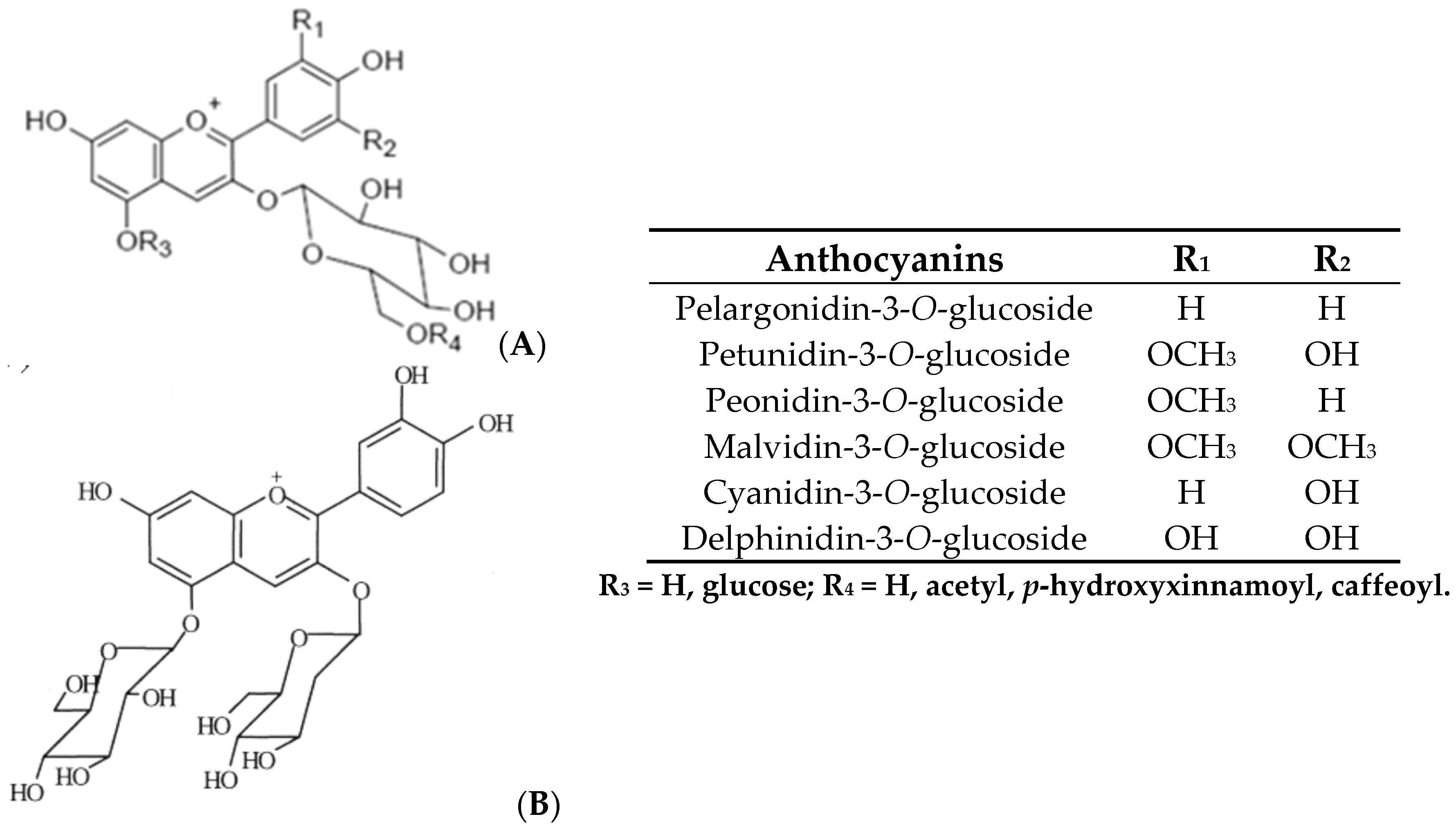
3. Biological Activity of Phenolic Compounds Present in Grape Juices
This entry is adapted from the peer-reviewed paper 10.3390/beverages4010022
References
- Rizzon, L.A.; Meneguzzo, J. Suco de Uva, 1st ed.; Embrapa Technological Information: Brasília, Brazil, 2007.
- Ali, K.; Maltese, F.; Choi, Y.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378.
- Rizzon, L.A.; Manfroi, V.; Meneguzo, J. Elaboração de Suco de uva na Propriedade Vitícola; Embrapa-CNPUV: Bento Gonçalves, Brazil, 1998; pp. 1–24.
- Camargo, U.A.; Maia, J.D.G.; Ritschel, P.S. Novas Cultivares Brasileiras de Uva; Embrapa Uva e Vinho: Bento Gonçalves, Brazil, 2010; 64p.
- Dutra, M.C.P.; Lima, M.S.; Barros, A.P.A.; Mascarenhas, R.J.; Lafisca, A. Influência da variedade de uvas nas características analíticas e aceitação sensorial do suco artesanal. Rev. Bras. Prod. Agroind. 2014, 16, 265–272.
- Soundararajan, R.; Wishart, A.D.; Rupasinghe, H.P.V.; Arcellana-Panlilio, M.; Nelson, C.M.; Mayne, M.; Robertson, G.S. Quercetin-3-glucoside protects neuroblastoma (SH-SY5Y) cells in vitro against oxidative damage by inducing SREBP-2 mediated cholesterol biosynthesis. J. Biol. Chem. 2008, 283, 2231–2245.
- Gollücke, A.P.B.; Catharino, R.R.; Souza, J.C.; Eberlin, M.N.; Tavares, D.Q. Evolution of major phenolic components and radical scavenging activity of grape juices through concentration process and storage. Food Chem. 2009, 112, 868–873.
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445.
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37.
- OIV—Organisation Internationale de la Vigne et du Vin. Vine and Wine Outlook; OIV: Paris, France, 2012; ISBN 979-10-91799-56-0.
- Soyer, Y.; Koca, N.; Karadeniz, F. Organic acid profile of Turkish white grapes and grape juices. J. Food Compos. Anal. 2003, 16, 629–636.
- Morris, J.R. Factors influencing grape juice quality. Hort Technol. 1998, 8, 471–478.
- Iyer, M.M.; Sacks, G.L.; Padilla-Zakour, O.I. Impact of harvesting and processing conditions on green leaf volatile development and phenolics in concord grape juice. J. Food Sci. 2010, 75, 297–304.
- Rizzon, L.A.; Miele, A. Características analíticas e discriminação de suco, néctar e bebida de uva comerciais brasileiros. Ciênc Tecnol Aliment. 2012, 32, 93–97.
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Identification of (Poly)phenolic Compounds in Concord Grape Juice and Their Metabolites in Human Plasma and Urine after Juice Consumption. J. Agric. Food Chem. 2011, 59, 9512–9522.
- Ribeiro, T.P.; Lima, M.A.C.; Alves, R.E. Maturação e qualidade de uvas para suco em condições tropicais, nos primeiros ciclos de produção. Pesq. Agrop. Bras. 2012, 47, 1057–1065.
- Camargo, U.A. ‘Isabel Precoce’: Alternativa Para a Vitivinicultura Brasileira. In Comunicado Técnico N° 54; Embrapa Uva e Vinho: Bento Gonçalves, Brazil, 2004.
- Camargo, U.A.; Maia, J.D.G. “BRS Cora” nova cultivar de uva para suco, adaptada a climas tropicais. In Comunicado Técnico N° 53; Embrapa Uva e Vinho: Bento Gonçalves, Brazil, 2004.
- Camargo, U.A.; Maia, J.D.G.; Nachtigal, J.C. “BRS Violeta” nova cultivar de uva para suco e vinho de mesa. In Comunicado Técnico N° 63; Embrapa Uva e Vinho: Bento Gonçalves, Brazil, 2005.
- Morris, J.R.; Striegler, K.R. Processing Fruits: Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005.
- Lima, M.S.; Dutra, M.C.P.; Toaldo, I.M.; Corrêa, L.C.; Pereira, G.L.; Oliveira, D.; Bordignon-Luiz, M.T.; Ninowd, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced in industrial scale by different processes of maceration. Food Chem. 2015, 188, 384–392.
- Monrad, J.K.; Suárez, M.; Motilva, M.J.; King, J.W.; Srinivas, K.; Howard, L.R. Extraction of anthocyanins and flavan-3-ols from red grape pomace continuously by coupling hot water extraction with a modified expeller. Food Res. Int. 2014, 65, 77–87.
- Kelebek, H.; Canbas, A.; Selli, S. Effects of different maceration times and pectolytic enzyme addition on the anthocyanin composition of Vitis vinifera cv. Kalecik karasi wines. J. Food Process. Preserv. 2009, 33, 296–311.
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 3, 781–784.
- Souquet, J.M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996, 43, 509–512.
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091.
- Fuleki, T.; Ricardo-Da-Silva, J.M. Effects of cultivar and processing method on the contents of catechins and procyanidins in grape juice. J. Agric. Food Chem. 2003, 51, 640–646.
- Shrikhande, A.J. Wine by-products with health benefits. Food Res. Int. 2000, 33, 469–474.
- Wroblewski, K.; Muhandiram, R.; Chakrabartty, A.; Bennick, A. The molecular interaction of human salivary histatins with polyphenolic compounds. Eur. J. Biochem. 2001, 268, 4384–4397.
- Auger, C.; Teissedre, P.L.; Gerain, P.; Lequeux, N.; Bornet, A.; Serisier, S.; Besançon, P.; Caporiccio, B.; Cristol, J.P.; Rouanet, J.M. Dietary wine phenolics catechin, quercetin, and resveratrol efficiently protect hypercholesterolemic hamsters against aortic fatty streak accumulation. J. Agric. Food Chem. 2005, 53, 2015–2021.
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Trattato di Enologia: Microbiologia del vino e Vinificazioni, 2nd ed.; Edagricole: Bologna, Italy, 2005.
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nunez, M.J. Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: Almond hulls and grape pomace. J. Agric. Food Chem. 2007, 55, 10101–10109.
- Jackson, R.S. Wine Science Principle and Applications, 3rd ed.; Academic Press: Burlington, VT, USA, 2008.
- Dopico-Garcia, M.S.; Fique, A.; Guerra, L.; Afonso, J.M.; Pereira, O.; Valentao, P.; Andrade, P.B.; Seabra, R.M. Principal components of phenolics to characterize red Vinho Verde grapes: Anthocyanins or non-coloured compounds? Talanta 2008, 75, 1190–1202.
- Vilela, A.; Cosme, F. Drink Red: Phenolic Composition of Red Fruit Juices and Their Sensorial Acceptance. Beverages 2016, 2, 29.
- Jin, Z.M.; He, J.J.; Bi, H.Q.; Cui, X.Y.; Duan, C.Q. Phenolic Compound Profiles in Berry Skins from Nine Red Wine Grape Cultivars in Northwest China. Molecules 2009, 14, 4922–4935.
- Cabrera, S.G.; Jang, J.I.H.; Kim, S.T.; Lee, Y.R.; Lee, H.J.; Chung, H.S.; Moon, K.D. Effects of processing time and temperature on the quality components of Campbell grape juice. J. Food Process. Preserv. 2009, 33, 347–360.
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, C.A.; Kuszynski, S.S.; Joshi, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197.
- Cantos, E.; Espin, J.C.; Tomas-Barberan, F.A. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J. Agric. Food Chem. 2002, 50, 5691–5696.
- Makris, D.P.; Kallithrakab, S.; Kefalasa, K. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404.
- Pace-Asciak, C.R.; Rounova, O.; Hahn, S.E.; Diamandis, E.P.; Goldberg, D.M. Wines and grape juices as modulators of platelet aggregation in healthy human subject. Clin. Chim. Acta 1996, 246, 163–182.
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 1–8.
- Sautter, C.K.; Denardin, S.; Alves, A.O.; Mallmann, C.A.; Penna, N.G.; Hecktheuer, L.H. Determinação de resveratrol em sucos de uva no Brasil. Ciênc. Tecnol. Aliment. 2005, 25, 437–442.
- Capanoglu, E.; Vos, R.C.H.; Hall, R.D.; Boyacioglu, D.; Beekwilder, J. Changes in polyphenol content during production of grape juice concentrate. Food Chem. 2013, 139, 521–526.
- Dani, C.; Oliboni, L.S.; Vanderlinde, R.; Bonatto, D.; Salvador, M.; Henriques, J.A.P. Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580.
- Lima, M.S.; Silani, I.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103.
- Toaldo, I.M.; Cruz, F.A.; Alves, T.L.; Gois, J.S.; Borges, D.L.G.; Cunha, H.P.; Silva, E.L.; Bordignon-Luiz, M.T. Bioactive potential of Vitis labrusca L. grape juices from the Southern Region of Brazil: Phenolic and elemental composition and effect on lipid peroxidation in healthy subjects. Food Chem. 2015, 173, 527–535.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956.
- Hidalgo, M.; Sanchez-Moreno, C.; Pascual-Teresa, S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696.
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of Muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503.
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415.
- Wada, M.; Kido, H.; Ohyama, K.; Ichibangas, T.; Kishikaw, N.; Ohba, Y.; Nakashima, M.N.; Kurod, N.; Nakashima, K. Chemiluminescent screening of quenching effects of natural colorants against reactive oxygen species: Evaluation of grape seed, monascus, gardenia and red radish extracts as multi-functional food additives. Food Chem. 2007, 101, 980–986.
- Marinova, E.M.; Yanishlieva, N.V. Antioxidant activity and mechanism of action of some phenolic acids at ambient and high temperatures. Food Chem. 2003, 81, 189–197.
- Coimbra, S.R.; Lage, S.H.; Brandizzi, L.; Yoshida, V.; da Luz, P.L. The action of red wine and purple grape juice on vascular reactivity is independent of plasma lipids in hypercholesterolemic patients. Braz. J. Med. Biol. Res. 2005, 38, 1339–1347.
- Dohadwala, M.M.; Vita, J.A. Grapes and Cardiovascular Disease. J. Nutr. 2009, 139, 1788–1793.
- Aviram, M.; Fuhrman, B. Wine flavonoids protect against LDL oxidation and atherosclerosis. Alcohol and Wine in Health and Disease. Ann. N. Y. Acad. Sci. 2002, 957, 146–161.
- Mudnic, I.; Modun, D.; Rastija, V.; Vukovic, J.; Brizic, I.; Katalinic, V.; Kozina, B.; Medic-Saric, M.; Boban, M. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010, 119, 1205–1210.
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220.
- Zunino, S. Type 2 Diabetes and Glycemic Response to Grapes or Grape Products. J. Nutr. 2009, 139, 1794–1800.
- Joseph, J.A.; Shukitt-Hale, B.; Willis, L.M. Grape Juice, Berries, and Walnuts Affect Brain Aging and Behavior. J. Nutr. 2009, 139, 1813–1817.
- Modun, D.; Music, I.; Vukovic, J.; Brizic, I.; Katalinic, V.; Obad, A.; Palada, I.; Dujic, Z.; Boban, M. The increase in human plasma antioxidant capacity after red wine consumption is due to both plasma urate and wine polyphenols. Atherosclerosis 2008, 197, 250–256.
- Ursini, F.; Sevanian, A. Wine Polyphenols and Optimal Nutrition. Ann. N. Y. Acad. Sci. 2002, 957, 200–209.
- Dávalos, A.; Fernández-Hernando, C.; Cerrato, F.; Martínez-Botas, J.; Gómez-Coronado, D.; Gómez-Cordovés, C.; Lasunción, M.A. Red Grape Juice Polyphenols Alter Cholesterol Homeostasis and Increase LDL-Receptor Activity in Human Cells In Vitro. J. Nutr. 2006, 136, 1766–1773.
- Castilla, P.; Echarri, R.; Davalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gomez-Coronado, D.; Ortuno, J.; Lasuncion, M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and anti-inflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006, 84, 252–262.
- Hernandez-Jimenez, A.; Gomez-Plaza, E.; Martinez-Cutillas, A.; Kennedy, J.A. Grape skin and seed proanthocyanidins from Monastrell x Syrah grapes. J. Agric. Food Chem. 2009, 57, 10798–10803.
- Novaka, I.; Janeiroa, P.; Serugab, M.; Oliveira-Brett, A.M. Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Anal. Chim. Acta 2008, 630, 107–115.
- Spáčil, Z.; Nováková, L.; Solich, P.; Spacil, Z.; Novakova, L.; Solich, P. Analysis of phenolic compounds by high performance liquid chromatography and ultra performance liquid chromatography. Talanta 2008, 76, 189–199.
- Natividade, M.M.P.; Corrêa, L.C.; Souza, S.V.C.; Pereira, G.E.; Lima, L.C.O. Simultaneous analysis of 25 phenolic compounds in grape juice for HPLC: Method validation and characterization of São Francisco Valley samples. Microchem. J. 2013, 110, 665–674.
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646.
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113.
- Carbonaro, M.; Mattera, M.; Nicoli, S.; Bergamo, P.; Capelloni, M. Modulation of antioxidant compounds in organic vs. conventional fruit (peach, Prunus persica L.; and pear, Pyrus commumis L.). J. Agric. Food Chem. 2002, 50, 5458–5462.
- Grinder-Pederson, L.; Rasmussen, S.E.; Bugel, S.; Jørgensen, L.V.; Dragsted, L.O.; Gundersen, V.; Sandström, B. Effect of diets based on foods from conventional versus organic production on intake and excretion o flavonoids and markers of antioxidative defense in humans. J. Agric. Food Chem. 2003, 51, 5671–5676.
