In line with consumer preferences and due to the effects of global climate change, new trends have emerged in wine fermentation and technology. Consumers are looking for wines with less ethanol and fruitier aromas but also with a good balance in terms of acidity and mouthfeel. Nonconventional yeasts contain a wide range of different genera of non-Saccharomyces. If they were considered spoilage yeasts in the past, they are used to enhance the aroma profile of wine or modulate wine composition.
- wine acidity
- Lactobacillus plantarum
- Lachancea thermotolerans
- Schizosaccharomyces pombe
- Candida stellate
- Torulaspora delbrueckii
- Zygotorulaspora florentina
- Pichia kudriavzevii
- Stermerella bacillaris
1. Acids Present in Grapes and Wines and Their Perceived Taste
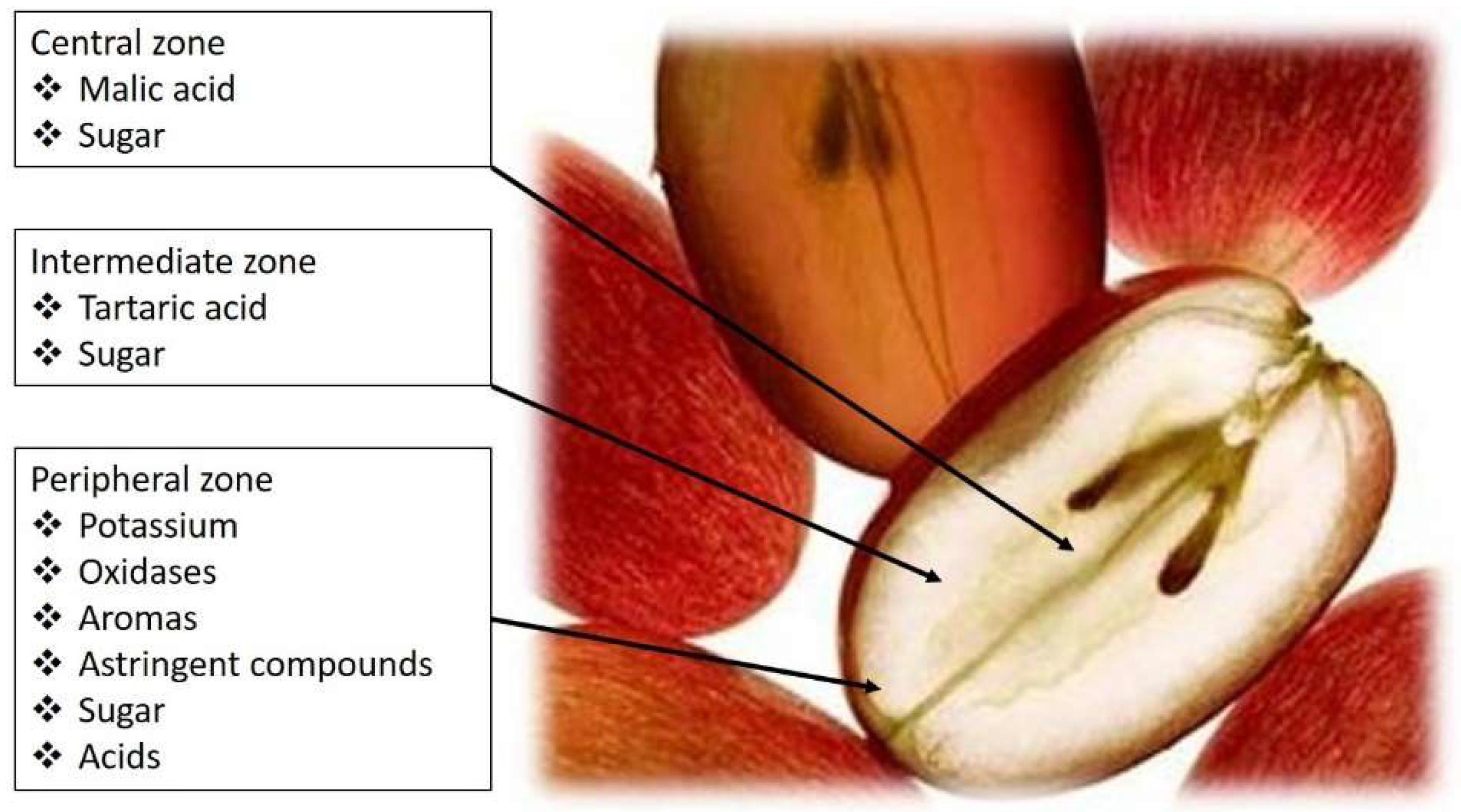
|
Fixed Acids |
Volatile Acids |
||
|---|---|---|---|
|
Major Acids |
Minor Acids |
Major Acids |
Minor Acids |
|
l-tartaric |
Amino-acids |
Acetic |
Formic |
|
(citrus-like taste) |
(vinegar-like) |
||
|
l-malic |
Pyruvic |
Propionic |
|
|
(metallic, green-apples taste) |
|||
|
l-lactic |
α-Ketoglutaric |
2-Methylpropionic |
|
|
(sour and spicy) |
|||
|
Citric |
Isocitric |
Butyric |
|
|
(fresh and citrus-like) |
|||
|
Succinic |
2-Oxoglutaric |
2-Methylbutyric |
|
|
(sour, salty, and bitter) |
|||
|
Dimethyl glyceric |
3-Methylbutyric |
||
|
Citramalic |
Hexanoic |
||
|
Gluconic acid (1) |
Octanoic |
||
|
Galacturonic |
Decanoic |
||
|
Glucuronic, Mucic, Coumaric, and Ascorbic |
|||
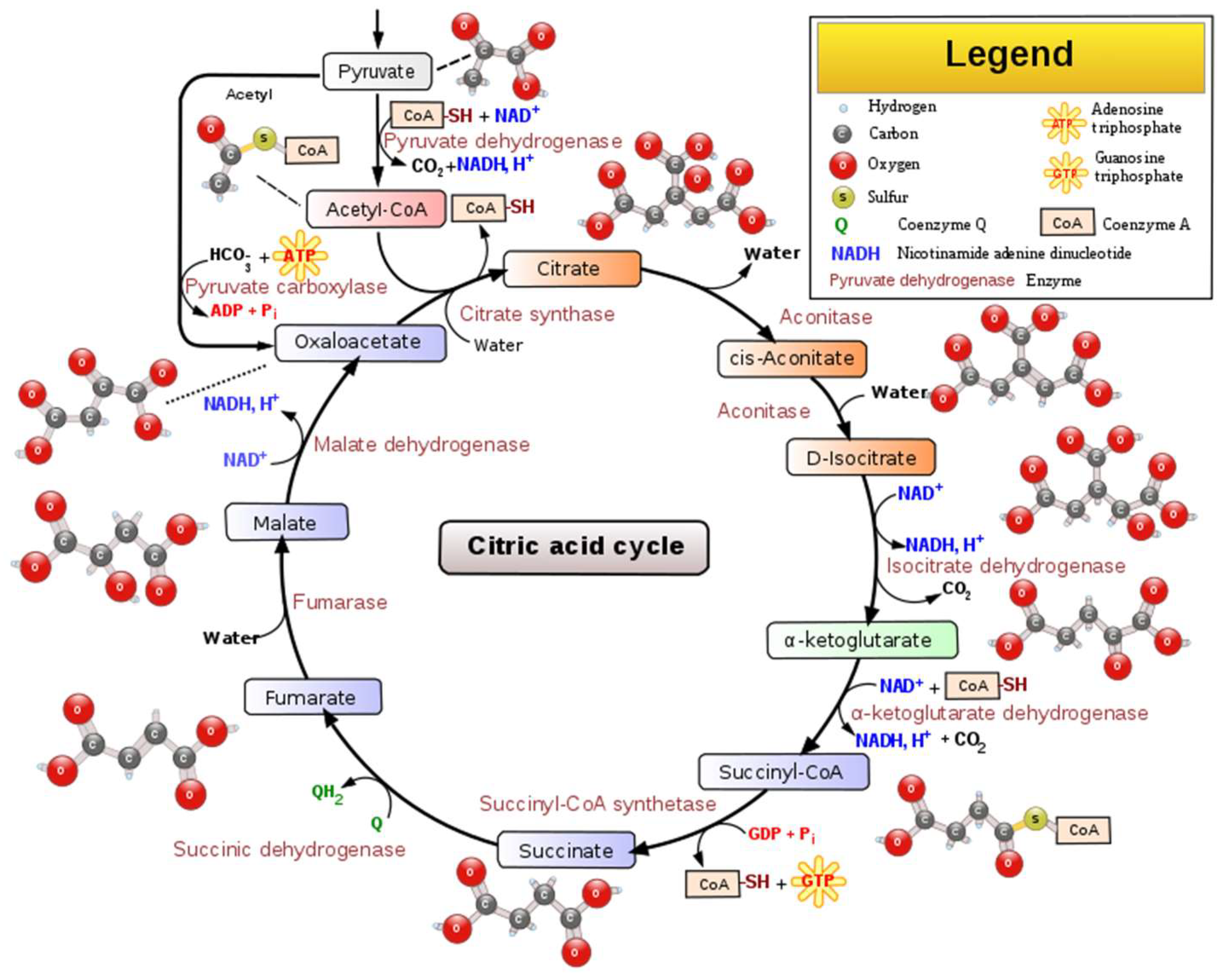
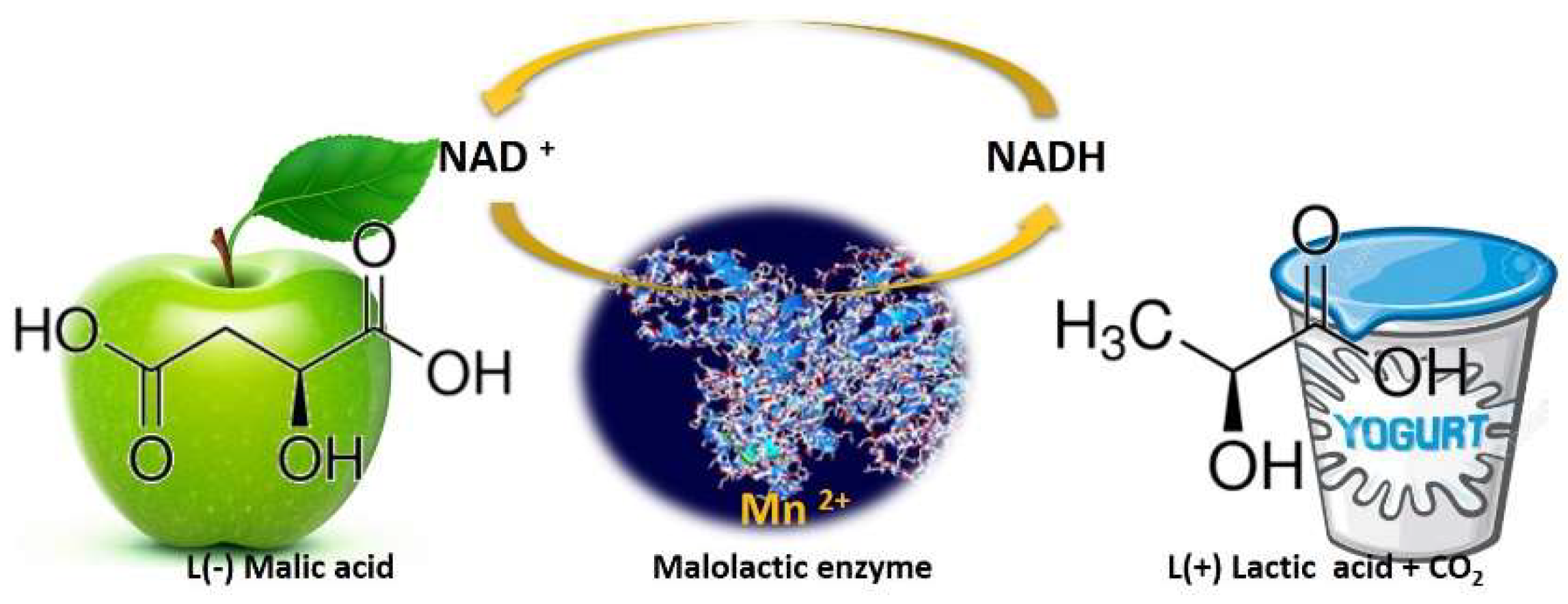
2. Wine Biological Acidity Modulation by Bacteria via Malolactic Fermentation
3. Wines Biological Acidity Modulation by Nonconventional Yeasts
|
Yeast Species |
Ethanol Formation (%, v/v) |
Sugars Fermented |
Volatile Compounds |
Effect on Wine Acidity |
Ref. |
|---|---|---|---|---|---|
|
Lachancea thermotolerans |
<9 |
Glucose Fructose Maltose Galactose |
2-phenyl ethyl acetate Ethyl lactate |
Acidity enrichment (lactic acid)/Acidity reduction (acetic acid) |
|
|
Schizosaccharomyces pombe |
12–14 |
Glucose Fructose Sucrose Maltose |
Higher alcohols Esters |
Maloalcoholic deacidification |
|
|
Candida stellate |
10.6 + 9.81 gL−1 glycerol (in co-culture with S. cerevisiae) |
Glucose Sucrose Raffinose (slow fermentation) |
Esters Acetoin |
Acidity enrichment (Succinic acid) |
[38] |
|
Torulaspora delbrueckii |
11 (table wine) 13-14 (i) (in co-culture with S. cerevisiae) |
Glucose Galactose (ii) Maltose (ii) Sucrose (ii) a,a-Trehalose (ii) Melibiose (ii) |
Long-chain alcohols, esters, aldehydes, and glycerol |
Low acetic acid production |
|
|
Z. florentinus/Z. Florentina |
>13 (iv) (in co-culture with S. cerevisiae) |
Fructose (iii) Glucose Galactose Sucrose Maltose Raffinose Trehalose |
higher alcohols and esters |
Low acetic acid production Some species are able to consume acetic acid (v) |
|
|
Pichia kudriavzevii/Issatchenkia orientalis |
>7 (vi) (in microvinifications with chemically defined grape juice) |
Glucose Fructose Sucrose, Maltose, Raffinose Xylose (vii) |
Esters and Higher alcohols |
Consume l-malic acid |
|
|
Starmerella bacillaris/Candida zemplinina |
11.7–12.1 (viii) |
Glucose Fructose (xix) |
Higher levels of some terpenes, lactones, and thiols.(x) |
Malic acid degradation; Reduction of acetic acid in sweet wines; Production of pyruvic acid. |
This entry is adapted from the peer-reviewed paper 10.3390/fermentation5010027
References
- Liu, H.F.; Wu, B.H.; Fan, P.G.; Xu, H.Y.; Li, S.H. Inheritance of sugars and acids in berries of grape (Vitis vinifera L.). Euphytica 2007, 153, 99–107.
- Cosme, F.; Gonçalves, B.; Inês, A.; Jordão, A.M.; Vilela, A. Grape and wine metabolites: Biotechnological approaches to improve wine quality. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; InTechOpen: London, UK, 2016; pp. 187–214.
- Amerine, M.A. The Maturation of Wine Grapes. Wines Vines 1956, 37, 53–55.
- Stafford, H. Distribution of Tartaric Acid in the Leaves of Certain Angiosperms. Am. J. Bot. 1959, 46, 347–352.
- Kliewer, W. Sugars and Organic Acids of Vitis vinifera. Plant Physiol. 1966, 41, 923–931.
- Lamikanra, O.; Inyang, I.; Leong, S. Distribution and effect of grape maturity on organic acid content of red Muscadine grapes. J. Agric. Food Chem. 1995, 43, 3026–3028.
- Ford, C.M. The Biochemistry of Organic Acids in the Grape. In The Biochemistry of the Grape Berry; e-Book, Gerós, H., Chaves, M., Delrot, S., Eds.; Bentham Science Publishers: Sharjah, UAE, 2012; pp. 67–88. ISBN 978-1-60805-360-5.
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Chapman and Hall: New York, NY, USA, 1996; pp. 102–181.
- Sharma, R.K. Citric Acid. In Natural Food Antimicrobial Systems; Naidu, A.S., Ed.; CRC Press LLC: New York, NY, USA, 2000.
- Citric Acid Cycle. Available online: https://commons.wikimedia.org/wiki/File:Citric_acid_cycle_with_aconitate_2.svg#/media/File:Citric_acid_cycle_with_aconitate_2.svg (accessed on 30 January 2019).
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Alcohols, and other volatile compounds. The chemistry of wine stabilization and treatments. In Handbook of Enology, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 2, pp. 51–64.
- Office Internationale de la Vigne et du Vin. International Code of Oenological Practices; OIV: Paris, France, 2010.
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.F.; Chaves, S.R.; Sousa, M.J.; Côrte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines—Minireview. Appl. Microbiol. Biotechnol. 2011, 89, 271–280.
- Coulter, A.D.; Godden, P.W.; Pretorius, I.S. Succinic acid-how is it formed, what is its effect on titratable acidity, and what factors influence its concentration in wine? Wine Ind. J. 2004, 19, 16–24.
- Hopfer, H.; Heymann, H. Judging wine quality: Do we need experts, consumers or trained panelists? Food Qual. Prefer. 2014, 32, 221–233.
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729.
- Dicks, L.M.T.; Dellaglio, F.; Collins, M.D. Proposal to reclassify Leuconostoc oenos as Oenococcus oeni gen. nov., comb. nov. Int. J. Syst. Bacteriol. 1995, 45, 395–397.
- Henick-Kling, T. Malolactic fermentation. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Reading, UK, 1993; pp. 289–327.
- Volschenk, H.; Van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136.
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549.
- Bartowsky, E.; Borneman, A. Genomic variations of Oenococcus oeni strains and the potential to impact on malolactic fermentation and aroma compounds in wine. Appl. Microbiol. Biotechnol. 2011, 92, 441–447.
- Lerm, E.; Engelbrecht, L.; du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212.
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae-Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004, 93, 141–154.
- Nehme, N.; Mathieu, F.; Taillandier, P. Impact of the co-culture of Saccharomyces cerevisiae-Oenococcus oeni on malolactic fermentation and partial characterization of a yeast-derived inhibitory peptidic fraction. Food Microbiol. 2010, 27, 150–157.
- Berbegal, C.; Peña, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological properties of Lactobacillus plantarum strains isolated from grape must fermentation. Food Microbiol. 2016, 57, 187–194.
- Lucio, O.; Pardo, I.; Krieger-Weber, S.; Heras, J.M.; Ferrer, S. Selection of Lactobacillus strains to induce biological acidification in low acidity wines. LWT Food Sci. Technol. 2016, 73, 334–341.
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; La Hens, D.V.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a malolactic starter culture in winemaking: A new (old) player? Electron. J. Biotechnol. 2019, 38, 10–18.
- Olguín, N.; Bordons, A.; Reguant, C. Multigenic expression analysis as an approach to understanding the behavior of Oenococcus oeni in wine-like conditions. Int. J. Food Microbiol. 2010, 144, 88–95.
- Miller, B.J.; Franz, C.M.; Cho, G.S.; du Toit, M. Expression of the malolactic enzyme gene (mle) from Lactobacillus plantarum under winemaking conditions. Curr. Microbiol. 2011, 62, 1682–1688.
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT Food Sci. Technol. 2016, 73, 557–566.
- Bravo-Ferrada, B.M.; Hollmann, A.; Delfederico, L.; La Hens, D.V.; Caballero, A.; Semorile, L. Patagonian red wines: Selection of Lactobacillus plantarum isolates as potential starter cultures for malolactic fermentation. World J. Microbiol. Biotechnol. 2013, 29, 1537–1549.
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166.
- Benito, A.; Calderón, F.; Palomero, F.; Benito, S. Combined use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523.
- Morata, A.; Suárez-Lepe, J.A. New biotechnologies for wine fermentation and ageing. In Advances in Food Biotechnology, 1st ed.; Ravishankar Rai, V., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2016; pp. 287–301.
- Vilela, A. Targeting Demalication and Deacetification Methods: The Role of Carboxylic Acids Transporters. Biochem. Physiol. 2017, 6, 224.
- Vilela, A. Lachancea thermotolerans, the Non-Saccharomyces Yeast that Reduces the Volatile Acidity of Wines. Fermentation 2018, 4, 56.
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50.
- Ferraro, L.; Fatichenti, F.; Ciani, M. Pilot scale vinification process using immobilized Candida stellata cells and Saccharomyces cerevisiae. Process. Biochem. 2000, 35, 1125–1129.
- Bely, M.; Stoeckle, P.; Masneuf Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii Saccharomyces cerevisiae culture on high sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320.
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015, 6, 1222.
- Pacheco, A.; Santos, J.; Chaves, S.; Almeida, J.; Leão, C.; Sousa, M.J. The Emerging Role of the Yeast Torulaspora delbrueckii in Bread and Wine Production: Using Genetic Manipulation to Study Molecular Basis of Physiological Responses. In Structure and Function of Food Engineering; Eissa, A.A., Ed.; IntechOpen: London, UK, 2012; Chapter 13; pp. 339–370.
- Lencioni, L.; Romani, C.; Gobbi, M.; Comitini, F.; Ciani, M.; Domizio, P. Controlled mixed fermentation at winery scale using Zygotorulaspora florentina and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2016, 234, 36–44.
- Lencioni, L.; Taccari, M.; Ciani, M.; Domizio, P. Zygotorulaspora florentina and Starmerella bacillaris in multistarter fermentation with Saccharomyces cerevisiae to reduce volatile acidity of high sugar musts. Aust. J. Grape Wine Res. 2018, 24, 368–372.
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180.
- Pina, C.; Gonçalves, P.; Prista, C.; Loureiro-Dias, M.C. Ffz1, a New Transporter Specific for Fructose from Zygosaccharomyces bailii. Microbiology 2004, 150, 2429–2433.
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of wines by selected yeast strains. Appl. Microbiol. Biotechnol. 2008, 80, 881–890.
- Mónaco, S.; Barda, N.; Rubio, N.; Caballero, A. Selection and characterization of a Patagonian Pichia kudriavzevii for wine deacidification. J. Appl. Microbiol. 2014, 117, 451–464.
- Hello, My Name is Pichia kudriavzevii; Eureka Brewing. Available online: https://eurekabrewing.wordpress.com/2014/02/16/hello-my-name-is-pichia-kudriavzevii (accessed on 16 February 2019).
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biotechnol. 2016, 100, 5515–5526.
- Masneuf-Pomarede, I.; Juquin, E.; Miot-Sertier, C.; Renault, P.; Laizet, Y.; Salin, F.; Alexandre, H.; Capozzi, V.; Cocolin, L.; Colonna-Ceccaldi, B.; et al. The yeast Starmerella bacillaris (synonym Candida zemplinina) shows high genetic diversity in winemaking environments. FEMS Yeast Res. 2015, 5, fov045.
- Englezos, V.; Giacosa, S.; Rantsiou, K.; Rolle, L.; Cocolin, L. Starmerella bacillaris in winemaking: Opportunities and risks. Curr. Opin. Food Sci. 2017, 17, 30–35.
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 2, 243–253.
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Fracassetti, D.; Ortiz-Julien, S.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360.