Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Emulsions and foams form the basis of an extensive variety of materials used in the beverage industry. One of the characteristics of beverage emulsions is that they are somewhat diluted, contain little amounts of a dispersed oil phase in the finished product, and must remain physically stable for long periods.
- colloids
- cream liqueurs
- soft drinks
- milk-coffee beverages
- functional beverages
- bottled water
- fruit drinks
- sparkling wine
- beer
1. Introduction
Colloidal systems are critical because they appear in various products and processes [1]. Food foams, emulsions, and suspensions form a significant part of the foods and beverages produced and sold today [2]. For instance, edible foams can be found in beer, bread, and chocolate mousse. Emulsions and foams form the basis of an extensive variety of materials used in food and drinks [3]. The main characteristics of beverage emulsions are that they are very dilute, containing as little as 20 mg/L of a dispersed oil phase in the finished product, and must remain physically stable for relatively long periods (4–12 months) [4]. One of the major concerns for dispersions is keeping the internal phase uniformly distributed during storage and consumption. This has led the food industry and many researchers to investigate the ability of hydrocolloids and proteins to stabilize emulsion and foam against creaming, flocculation, coalescence, drainage, and coarsening, depending on their intended application [5]. The use of food emulsifiers began in 1869 in France. The French chemist Hippolyte Mège-Mouriès produced Margarine, a substitute for butter, made by emulsifying milk with lipids [6]. The beverage industry soon employed this technique.
Citrus flavor (essential oils extracted from the orange or lemon peel) is one of the most popular flavors used in soft drink formulation. Since they are oily substances (insoluble in water), the drinks made with these oils are diluted oil/water (O/W) emulsions.
Different kinds of beverage colloids are presented in Figure 1. Due to their intended use, food colloids need to be non-toxic, non-carcinogenic, and non-allergenic. They must also be stable for months to years, including stability against destabilization processes such as aggregation, creaming, coalescence, and gelation. Innovative ingredients and solutions are emerging to help formulators add flavors, colors, and healthy ingredients and keep up with consumer demands for innovative beverages [6]. Chocolate drinks provide a related example of a beverage suspension.

Figure 1. Examples of beverage emulsions (O/W)—Oil and water are immiscible, but with the addition of stabilizing agents, an emulsion can be achieved (A); and foams suspensions beverages—foam consists of a mass of tiny bubbles that are formed when air and a liquid are mixed (B).
2. The Colloidal State
The word “colloid” has been used since 1862 to classify all the liquids that are colloidal dispersions. Chocolate, milk, and mayonnaise are examples of food colloids. Indeed, the colloidal state was recognized by Thomas Graham in 1861. This researcher observed two classes of substances:
- -
-
Crystalloid: readily pass through animal and vegetable membranes;
- -
-
Colloids: diffused very slowly and cannot pass through membranes.
Fifty years later, the colloidal state was described by Wolfgang Ostwald as a “world of neglected dimensions,” a reference to the world of systems in which the particles are microscopic [7]. Thus, a suspension of tiny particles in a continuous phase, the dispersion medium, is called a colloidal dispersion. The suspended particles are single large molecules or aggregates of molecules or ions ranging in size from 1 to 1000 nm [8]. Since a colloidal solution or substance comprises scattered particles, light cannot pass straight through. This effect was observed and described by John Tyndall as the Tyndall Effect. The Tyndall effect is an easy way of determining whether a mixture is colloidal (Figure 2).

Figure 2. The Tyndall effect [9]. When light is shined through a proper solution, it passes cleanly through the solution; however, when it is passed through a colloidal solution, the substance in the dispersed phases scatters the light in all directions, making it readily seen.
3. Classification of Emulsions, Foams, and Suspensions
3.1. Emulsions
Emulsions are colloidal dispersions in which a liquid is dispersed in a continuous liquid phase of different compositions [1,7]. Several classes of emulsions may be distinguished: oil-in-water (O/W) for oil droplets dispersed in water, water-in-oil (W/O) for water droplets dispersed in oil [1], and water-in-water (W/W) for two immiscible aqueous phases that are in thermodynamic equilibrium [15,16]. The W/W emulsions are very interesting colloidal dispersions much less known than conventional oil-in-water or water-in-oil emulsions, even though phase separation in aqueous mixtures is widespread. This emulsion could result from combinations of hydrophilic polymers [15]. Dilution tests may be carried out to find out whether an emulsion is oil-in-water or water-in-oil. These consist of a microscopic observation of a slide where a small amount of either oil or water is added to the emulsion sample [17]. It is also possible to formulate emulsions without water. Non-aqueous or anhydrous emulsions could be developed as O1/O2 systems or oil in a polar solvent emulsion, as well as multiple oil-in-oil-in-oil systems or variants [7].
Most emulsions are not thermodynamically stable; they tend to separate into “oil” and “water” phases. Therefore, an emulsifying agent is usually required to form a stable emulsion [1,17].
Most meta-stable emulsions encountered in practice contain oil, water, and an emulsifying agent (or stabilizing agents), usually a surfactant, macromolecule, or finely divided solids. The emulsifying agent, or protective colloid, is surface-active, meaning that it reduces the surface tension of the liquid and so tends to concentrate in boundary films [17].
3.2. Foams
A foam is a colloidal dispersion in which a gas is dispersed in a continuous liquid phase [1]. Foams are somewhat different from emulsions because, in foams, it is the dispersion medium that has colloidal dimensions [13]. Even though the bubbles in persistent foams are polyhedral and not spherical, it is conventional to refer to the “diameter” of gas bubbles in foams as if they were spherical. Foam bubbles usually have diameters more significant than 10 µm and may be larger than 1000 µm [1]. According to Calvert [17], a colloidal adsorptive agent forms a film that bounds the gas bubbles in liquid foam. The colloidal dimension in a foam is the film's thickness, not the bubble's size. The bubble is lighter than its surroundings and will rise to the top, where it joins the foam. When carbon dioxide is released, it uses the adsorptive agent to make the foam. Meringue is a dried foam using egg albumin. Marshmallows use sugared gelatin for the same purpose.
3.3. Suspensions
A suspension is a colloidal dispersion in which a solid is dispersed in a continuous liquid phase [1]. On the other hand, Florence and Attwood [7] define suspensions as dispersions of an insoluble substance in an aqueous or non-aqueous continuous phase. Suspensions may be either aqueous or non-aqueous. The particles in suspensions are more significant than 1000 nm, according to Schramm [1], usually have diameters greater than 0.2 µm, a spherical shape, and may be visible to the naked eye or under a microscope.
If a suspension contains, in addition to solid particles and a continuous liquid phase, emulsified droplets, and gas bubbles, two situations may occur: (i) If the solid particles are adsorbed on the emulsion droplets, then it is an emulsion that also contains solids; and (ii) if the particles and droplets are not mutually associated, then the system is at once a suspension and an emulsion. In these situations, the term used should be the one that best fits the context. Frequently, one or the other is considered the primary dispersion, while the different phase is regarded as an additive or a contaminant [1].
4. Recent Developments in Beverage Emulsions
4.1. Cream Liqueurs
Alcoholic emulsion creams, or cream liqueurs, are sweet semi-liquid alcoholic drinks that contain over 400 g/L of total extract and are often 17% (v/v) in alcohol or as high as 25% (v/v). Examples of alcoholic spirits used are brandy, whiskey, and vodka [18]. Most products contain several other added ingredients, which may include sugar, full-fat milk powder, non-fat milk solids, flavorings, colorings, preservatives, and a thickening agent such as sodium caseinate, which also acts as a stabilizing agent to prevent the cream and alcohol from separating [1]. Examples of cream liqueurs are Irish Baileys and Carolans Scotch Heather (made with whiskey). French chocolate rum Caprice, Dutch Bols, Italian and Czech liqueurs, and Polish egg-vanilla Advocaat belong to that group [19].
Cream liqueurs are beverage emulsions where petite droplet sizes are needed to form the emulsion, so a coarse emulsion is usually passed through a high-pressure homogenizer [1,20], which can produce tiny droplets (≈2 µm in diameter). High shear rates and high flow are needed for emulsification into small droplets and for good heat transfer or blending, respectively [1,21].
4.2. Soft Drinks
Soft drinks are called “soft” compared to “hard” alcoholic beverages. In a soft drink, small amounts of alcohol may be present, but the alcohol content must be less than 0.5% (v/v) of the total volume if the glass is to be considered non-alcoholic. Thus, “soft drink” applies to beverages containing flavorings, fruit juices, and other technological or nutritional value constituents. A soft drink typically contains a sweetener and a flavoring. The sweetener may be fruit juice, sugar, high-fructose corn syrup, and sugar substitutes in the case of diet drinks. These drinks may also contain caffeine, colorings, preservatives, and other ingredients [24]. To meet current stringent quality and legislative controls, before going to the market, a new beverage is subjected to extensive trials to assess the suitability, performance, and quality of all its components, and as there are millions of liters sold per year, it is essential to reach the correct formula to achieve a product that could be reproducible.
Soft drinks may be served chilled or at room temperature. Occasionally, soft drinks, such as Dr. Pepper, can be served warmly [25]. Soft drinks are available to consumers in different containers, including cans, glass, and plastic bottles, in various sizes ranging from small bottles to large 2-liter containers.
The consumption of sugar-sweetened beverages (SSBs) is associated with obesity [26], type 2 diabetes (T2D) [27], dental caries, and low nutrient levels. It could also be associated with many weight-related diseases, including diabetes, metabolic syndrome, cardiovascular risk factors, and elevated blood pressure [28]. According to Sen [24], experimental studies support a causal role of sugar-sweetened soft drinks in these illnesses. “Sugar-sweetened” includes drinks that use high-fructose corn syrup, as well as those using sucrose. Many soft drinks contain ingredients that are themselves sources of concern, like caffeine, which is linked to anxiety and sleep disturbance. Bhupathiraju and co-workers [29] found that the association between higher SSBs consumption and a higher risk of T2D was potentially a result of the high content of sucrose or high fructose and not of a joint effect of caffeine and sugar. These findings are significant because substituting caffeinated SSBs with caffeine-free SSBs in the human diet will not likely decrease the risk of T2D. Instead, the results suggest that replacing SSB with coffee or tea may lower the risk of T2D.
Soft drinks mix dry ingredients and fruit juices with water. Production of soft drinks can be done at factories or simply at home by mixing either syrup or dry ingredients with carbonated water. Carbonated water is made using a soda siphon or by dropping dry ice into water [30].
4.3. Milk-Coffee and Rich-Milk-Coffee Beverages
Milk coffee beverages contain about 1% milk fat, forming an oil-in-water emulsion. To emphasize the coffee flavor, many milk coffee beverages contain coffee extracts, the so-called “rich milk coffee” beverages. When the content of the coffee extracts increases, milk coffee beverages become unstable. Since the storage period for these milk coffee beverages is 6 to 12 months, the milk fat gradually floats towards the air–emulsion interface. Aggregation or flocculation [35,36] follows, and a milk ring is formed at the air–emulsion interface. The milk ring formation, or “oiling off,” is accelerated in these drinks. Moreover, the average drop size distribution of the combined emulsifier system containing protein (in this case, milk proteins) increases because the protein induces an attractive depletion interaction between droplets, thereby inducing flocculation and coalescence [37]. The transparent plastic bottle aggravates this situation: consumers can see that the milk fat has separated, so the product value depreciates. When flocculation happens, even if the milk coffee beverage is repeatedly shaken, the milk ring will not re-disperse, and some lumps of the milk fat will float on the surface. As explained by Ogawa and Cho [38], if the milk fat sticks to the can or plastic bottle, consumers will feel that these beverages are of low quality and will no longer purchase them. These authors [36] found that excessive roasted coffee beans will make the milk coffee unstable. Adding emulsifiers, like polyglycerol esters with long hydrophilic moieties, is practical for ensuring the emulsion’s stability.
4.4. Beverage Concentrates
Beverage concentrates are products sold in fluid or powdered form, which consumers then dilute with water before consumption. These formulations may contain ingredients, such as flavors, sweeteners, color additives, nutrients, or others, which form a drinkable beverage after dilution. According to Klein and co-workers [39], beverage concentrates, also known as “cloudy emulsions,” are usually prepared as concentrates and diluted later to the final product. The cloudy emulsion provides flavor, color, and cloud (turbidity) to the soft drink.
Due to ingredients present in a highly concentrated form (high acid, sweetener, flavor, buffer, and nutraceuticals), beverage concentrates can suffer spoilage, which reduces shelf-life once accelerated chemical reactions occur [40]. Thus, they must be stabilized by emulsifiers and amphiphilic polysaccharides such as whey protein isolate and Arabic gums, which, in aqueous solution, interact via electrostatic force and improve the emulsion stability [39].
4.5. Functional Beverages
The functional beverage category includes isotonic (beverages that rehydrate), lifestyle/wellness drinks, meal replacements, and medicinal teas. There is an increasing consumer demand for products beneficial to health and human wellness [40]. There is a high demand to fortify many beverage products with ingredients consumers perceive as giving added health benefits, such as vitamins, proteins, antioxidants, minerals, dietary fibers, and ω-3 fatty acids [40].

4.6. Bottled Water
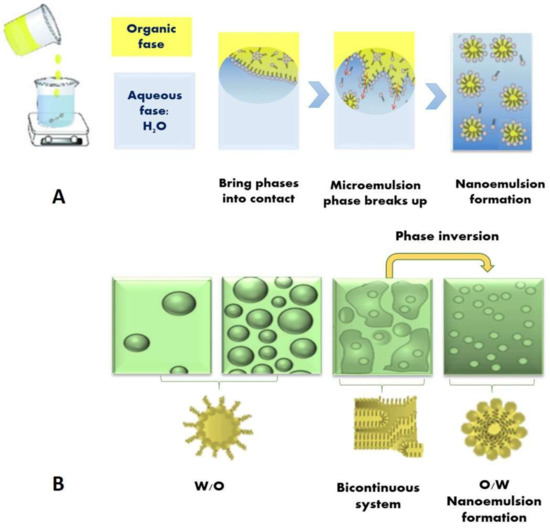
Recently, there has been an increase in the global sales of flavored bottled waters and fruit-flavored waters. Utilization of conventional emulsions in bottled waters to deliver oil-soluble flavors and nutraceuticals is limited due to the increase in turbidity caused by light scattering from the oil droplets [48]. However, microemulsions and nanoemulsions that contain very fine droplets that do not scatter light intensely may be used for this purpose [57] (Figure 3). Microemulsions are potentially excellent carriers for bioactive molecules. They offer the advantage of spontaneous formation, ease of manufacture, thermodynamic stability, and improved solubilization of bioactive materials. Spernath and Aserin [58] found that even W/O microemulsions, which are expected to break upon dilution in the digestive tract, increase the permeability and bioavailability of drugs.

Figure 3. Schematic diagram of a potential mechanism for forming nanoemulsions: (A) by the spontaneous emulsification method. When the organic phase (oil + hydrophilic surfactant) and aqueous phase (water) are brought into contact, a bicontinuous microemulsion is formed at the boundary, which breaks up and forms tiny oil droplets; (B) by the emulsion phase inversion method. In this case, water is titrated into a surfactant–oil–oil mixture with constant stirring.
4.7. Fruit Drinks
Today’s consumer demand for beverages with added health benefits adds another complexity to the formulation and development process. The beverage industry responds to these requests by reducing calories and minimizing ingredient lists. For the consumer, a beverage communicates functionality by its cloudy appearance and thickness in the mouth [59]. To invoke the idea of freshly squeezed juice, fruit juices incorporate specific cloud emulsions in product formulations, suggesting that the juice is made of ripe, natural fruit and vegetable ingredients [59].
Xanthan gum (Figure 4) is a polysaccharide used as a food additive. It is a powerful thickening agent and could be used as a stabilizer. It can be produced from a range of simple sugars using a fermentation process performed by a strain of Xanthomonas campestris. Xanthan gum is used in fruit-flavored drinks as a stabilizer for odor and flavor, and at the same time, it contributes to the texture.

Figure 4. Powdery form of xanthan gum and carboxymethyl cellulose (CMC).
4.8. Sparkling Wine and Beer
Sparkling wine undergoes a second alcoholic fermentation to induce carbonation and the consequent creation of bubbles. These bubbles (foam) are short-lived and not very stable. However, it is thought that proteins, due to their surfactant properties, and polysaccharides from the grapes used for wine-making are essential factors in foam formation and stability [63,64,65].
The foam formation and foamability parameters seem to be dependent on (1) the variety of grape used due to differences in the concentrations and types of proteins; (2) aging time and contact time with the yeast of the second fermentation due to the release of glycoproteins, yeast mannoproteins released during fermentation and autolysis that have been described as the significant foam promoters due to their structure, which favors adsorption to the foam bubbles gas/liquid interface [64,66]; (3) concentration of iron in the wine, due to the ability of iron cations to complex with the proteins [67]; (4) wine anthocyanins (mainly different forms of malvidin) and amino acids (mainly β-alanine that confer hydrophobicity to the peptides and improve the quality of the foam) [65].
Consumer preferences for beer foams vary but can be characterized in terms of foam stability, quantity, lacing (adhesion to a glass surface), whiteness, “creaminess” (bubble texture), and concentration. The foam “head” created when the beer is poured or dispensed is also essential to consumer approval of a particular beer product. Also, unlike sparkling wine, whose foam film lifetimes are short (hydrodynamic control), beer foam has a slower drainage rate due to the adsorption of proteins at the interfaces and the generation of a significant disjoining pressure between bubbles [1].
Beer foam formation and stability are influenced by raw materials, namely malt and brewing. Evans et al. [68] established that the foam stability depends on the malt source. However, the “head” character cannot be predicted. According to several authors, the beer foam stability is determined by the interaction of several components present in beer, mainly proteins/polypeptides originating from malt and iso-α-acids from hop [69,70,71].
Another consumer-driven objective, in addition to stable foam, is the so-called “lace curtains” on the wall of glass and cling patterns. The adhesiveness needed by the foam to produce this effect appears to be derived from the hops [71].
This entry is adapted from the peer-reviewed paper 10.3390/beverages4020025
This entry is offline, you can click here to edit this entry!
