Methylxanthines (MTX) are purine derived xanthine derivatives. Methylxanthines are also known to have anti-inflammatory and anti-oxidative properties, mediate changes in lipid homeostasis and have neuroprotective effects.
- methylxanthines
- Parkinson´s disease
1. Introduction
Neurodegenerative disorders share several common histopathological hallmarks and mechanisms, like neuronal cell loss linked to gliosis, misfolding and accumulation of proteins, oxidative stress, and neuroinflammation. In case of AD, disease progression is strongly associated with Aβ peptide generation and aggregation, associated with pathological extracellular and intracellular filamentous deposits, hyperphosphorylated tau proteins, neuroinflammation and synaptic loss. PD is characterized by dopaminergic neurodegeneration and accumulation of α-synuclein in Lewy bodies. Also, in patients with PD, Lewy bodies are seen in the substantia nigra, the basal nucleus of Meynert, the locus ceruleus, the cerebral cortex, the sympathetic ganglia, the dorsal vagal nucleus, the myenteric plexus of the intestines, and even in the cardiac sympathetic plexus.[1] Concerning MS, neuroinflammatory processes leading to demyelination of neurons are in the focus of the disease.
Numerous studies have been performed to address the question of whether xanthine derivatives like caffeine and theobromine have beneficial properties in respect to the characteristic histopathological changes that occur in the above-mentioned diseases and to cognitive decline. Although the outcome of the clinical studies, especially for AD, were heterogeneous [2], some mechanisms were identified, showing how methylated xanthine derivatives could protect against neuronal damage.
In general, in physiological concentrations, achieved for example by coffee consumption or by intake of methylxanthine containing beverages, methylxanthines act as antagonists of the adenosine receptor (AR), histone deacetylase activator or antioxidant. Affecting these pathways, xanthine derivatives are able to modulate molecular mechanisms associated with neurodegenerative diseases like accumulation of misfolded proteins, oxidative stress and neuroinflammation. Interestingly, in particular the beneficial role of adenosine receptor antagonists in the treatment of neurodegenerative diseases has become more and more apparent in the last years [3][4][5][6].
2. Parkinson’s Disease
2.1. Epidemiological and Clinical Studies
Parkinson´s disease (PD) is one of the most common neurodegenerative diseases characterized by a loss of dopaminergic-neurons in the substantia nigra and accumulation of misfolded α-synuclein protein aggregates in Lewy bodies. Leading symptoms of PD are bradykinesia or akinesia with rest tremor, postural instability or rigor. Additionally, patients suffering from PD show a broad range of non-motor symptoms differing in early and late-stage symptoms, e.g., obstipation and dementia. Despite an unknown etiology of PD, many risk factors such as age, male gender, environmental factors, intestinal inflammation, and genetics are described [7][8]. Besides the known risk factors it is discussed that nutrition may be associated with increased (dairy products) or decreased (phytochemicals, Omega-3 fatty acids, tea) risk or progression in PD [9]. Multiple epidemiological studies could already demonstrate an inverse association between coffee drinking and PD and thereby suggest a link to caffeine, and consequently methylxanthines, as A2A receptor antagonists [10][11][12]. Recent studies have confirmed this association (for an overview see Table 1). Bakshi et al. found a significantly lower caffeine intake, determined through questionnaire, in idiopathic PD patients compared to control group from the Harvard Biomarkers Study cohort with 369 cases of PD and 197 healthy controls [13]. In a study from Fujimaki et al., the authors investigated serum from 108 patients with PD by liquid chromatography-mass spectrometry (LC-MS) compared to 31 age-matched healthy control participants and found significantly lowered serum levels of not only caffeine, but also its downstream products like theophylline, theobromine and paraxanthine [14]. Crotty et al. demonstrated similar results in a recent metabolomics study showing a significantly lower plasma and CSF concentration of caffeine (71% lower) and its degradation products, e.g., paraxanthine and theophylline (57%, 56% lower), in PD patients versus control group measured via LC-MS. Subgroup analysis of carriers of the LRRK2 mutation, a mutation in the leucine-rich repeat kinase 2 gene (known for an increased risk for sporadic PD) [15], revealed an even greater decrease of plasma caffeine level by 76% for PD LRRK2 mutation carriers compared to healthy LRRK2 carriers [16]. Ohmichi et al. found similar results for the methylxanthine analogue theophylline and confirmed a significantly lower plasma concentration in patients with PD versus an age-matched control group [17]. A recent meta-analysis by Hong et al., which included 13 studies in total, showed that for healthy individuals, caffeine consumption results in a significantly lower risk of developing PD specific symptoms (HR = 0.797, 95% CI: 0.748–0.849, p < 0.001, I2: ~ 15.41%, 9/13 studies). Furthermore, a significant deceleration of PD progression among early-stage PD individuals with higher caffeine consumption was found (HR = 0.834, 95% CI = 0.707–0.984, p = 0.03, I2: ~39.7%, 4/13 studies) [18]. Another interesting recent study, carried out by Maclagan et al., used a computational approach to rank 620 drugs with the ability to inhibit α-synuclein aggregation. They examined associations between the top 15 drugs in case-control validation study by using health administrative databases. Their logistic regression models found that patients exposed to methylxanthines, especially the synthetic pentoxifylline (PTX) and the natural occurring theophylline, were associated with decreased odds of occurrence of PD. Additionally longer durations of PTX administration revealed a trend towards dose-response [19]. One modified methylxanthine, istradefylline (ISD), has been used to decrease daily “off episodes”, a state where symptoms recur when the effect of L-DOPA weakens, in PD patients. ISD was approved first in Japan in May 2013 and by the FDA in the US in 2019 [20]. A meta-analysis including six studies revealed that treatment with ISD 40 mg/day decreased the duration of “off episodes” and enhanced the motor symptoms of PD patients. Similar effects could be found for 20 mg/day. ISD treatment revealed no significant effect on adverse effects. In summary the meta-analysis showed that ISD 20 mg and 40 mg both improved the unified Parkinson’s disease rating scale III (UPDRS) [21].
Table 1. Summary of recent clinical studies investigating the relationship between methylxanthines and Parkinson´s Disease (PD). n: sample size; CSF: cerebrospinal fluid.
| Author | Year | Type of Study/n | Substance | Outcome |
|---|---|---|---|---|
| Bakshi et al. [13] | 2020 | cross sectional, case-control/197 healthy control vs. 369 idiopathic PD patients | caffeine, urate | the authors found a robust inverse association between idiopathic PD and caffeine intake and urate plasma levels |
| Fujimaki et al. [14] | 2018 | clinical trial/31 healthy control vs. 108 PD patients without dementia1 | caffeine, theophylline, theobromine, paraxanthine and other downstream metabolites | absolute lower levels of caffeine and metabolites were found to be a promising biomarker for early PD |
| Crotty et al. [16] |
2020 | clinical trial/samples from “23andMe” study, LRRK2 longitudinal study and LRRK2 cross-sectional study (n = 380) | caffeine, theophylline, paraxanthine and other downstream metabolites, trigonelline (non-xanthine constituent of coffee) | significantly lower plasma and CSF levels of caffeine and downstream metabolites in PD patients, even more in LRRK2 mutation carriers |
| Ohmichi et al. [17] | 2018 | clinical trial/31 PD patients vs. 33 age-matched controls | theophylline | theophylline serum levels were significantly lower in PD patients compared to control |
| Hong et al. [18] |
2020 | meta-analysis/13 studies (9 healthy cohort, 4 PD cohort) | caffeine | caffeine consumption resulted in a significantly lower rate of PD |
| Maclagan et al. [19] | 2020 | computational & pharmacoepidemiologic study ranking 620 drugs, case-control study/14,866 PD and 74,330 controls | pentoxifylline, theophylline, dexamethasone | the authors state, that corticosteroids and the found methylxanthines should be investigated as disease-modifying drugs |
| Sako et al. [21] |
2017 | meta-analysis/six studies | istradefylline | 20 and 40 mg/day of istradefylline revealed significantly decreased durations of “off episodes” in PD patients |
2.2. Animal Studies/Molecular Pathways
Older studies have already demonstrated neuroprotective effects of caffeine in PD mouse models [22]. In line with these previous encouraging results, Luan et al. demonstrated that chronic caffeine treatment reduced the effect of intra-striatal injected human A53T α-Synuclein fibrils in mice [23]. Caffeine was administered seven days before the injection and applied for 120 days at concentrations of ~0.4–2 mg/L. They found significantly decreased inclusion of α-Synuclein in the striatum of the caffeine treated mice. Furthermore, apoptosis, microglial activation and astrogliosis were significantly reduced [23]. A similar study used 52 rats with a control group, a PD model group—generated by injecting 1.5 mg/kg rotenone intraperitoneally (i.p.) for 45 days—and two caffeine groups. One of the caffeine groups was injected with 30 mg/kg caffeine i.p. in addition to the administered rotenone for 45 days, whereas the other group was treated with caffeine after the induction of PD via rotenone. The study revealed that co-treatment and post-treatment with caffeine in the intoxicated rats enhanced the dysfunction of rotenone-induced motor symptoms by recovering dopamine levels in the midbrain and striatum. In addition, caffeine improved the antioxidant effect by reducing rotenone-induced lipid peroxidation and superoxide dismutase activity in the striatum and midbrain. Furthermore, the data revealed reduced rotenone-induced TNF-α activity in the caffeine groups indicating an anti-inflammatory effect of caffeine. Caffeine protection and treatment restored open field test parameters, forelimb hanging test and traction test to nearly control group values. Histopathological investigation revealed a degeneration of neurons and presence of Lewy bodies in the rotenone-induced PD group, which was prevented in both caffeine groups with a more prominent effect in the caffeine protected group [24]. Pardo et al. demonstrated in another animal study that the naturally occurring methylxanthine theophylline can reverse motor symptoms in rats. In this study theophylline reversed locomotion, catalepsy and tremulous jaw movement (resembling parkinsonian tremor) induced by pimozide, a D2 dopamine antagonist [25]. In conclusion, these studies show a link for caffeine and other methylxanthines acting as A2A receptor antagonists with a decreased risk for PD and modification of progression, indicating new adenosine receptor ligands might be encouraging targets in treating PD. In a study by Rohilla et al. newly synthesized xanthine derivates as selective AR antagonists were analyzed. Evaluation of the antiparkinsonian effect in the study was carried out by inducing catatonia in rats with perphenazine. Most xanthines significantly lowered the catatonic score compared to control. The most potent antiparkinsonian effect was found for the methylxanthine RB-531 (8-[3-(3-Chloropropxy)]-1,3-dipropyl-7-methylxanthine), showing a similar response as the standard treatment L-DOPA [26]. The abovementioned studies and their outcomes are summarized in Figure 2 and Table 2.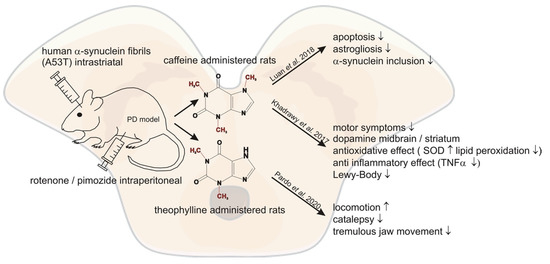 Figure 2. Summary of the molecular mechanisms reported in recent literature potentially mediating the beneficial effects of methylxanthines in respect to Parkinson´s disease.
Figure 2. Summary of the molecular mechanisms reported in recent literature potentially mediating the beneficial effects of methylxanthines in respect to Parkinson´s disease.
Table 2. Summary of recent animal and cell culture studies investigating the relationship between methylxanthines and Parkinson´s disease (PD).
| Author | Year | Used Model | Substance | Outcome |
|---|---|---|---|---|
| Luan et al. [23] | 2018 | Injected α-synuclein fibrils intra-striatal in mice | caffeine | reduced inclusion of α-synuclein, apoptosis, microglial activation and astrogliosis after caffeine treatment |
| Khadrawy et al. [24] | 2017 | rotenenoe induced PD mice model | caffeine | recovering dopamine levels in midbrain and striatum ameliorating motor symptoms, antioxidative and anti-inflammatory effect of caffeine, prevention of neurodegeneration through less lewy bodies |
| Pardo et al. [25] | 2020 | pimozide induced PD mice model | theophylline | reversed locomotion, catalepsy and tremulous jaw movement |
| Rohilla et al. [26] | 2019 | perphenazine induced catatonia in rats | newly synthesized xanthine derivatives | most xanthines significantly lowered catatonia score, most potent MTX shows a similar response as L-DOPA |
This entry is adapted from the peer-reviewed paper 10.3390/nu13030803
References
- Koichi Wakabayashi; Kunikazu Tanji; Saori Odagiri; Yasuo Miki; Fumiaki Mori; Hitoshi Takahashi; The Lewy Body in Parkinson’s Disease and Related Neurodegenerative Disorders. Mol. Neurobiol. 2012, 47, 495-508, .
- Wasim, S.; Kukkar, V.; Awad, V.M.; Sakhamuru, S.; Malik, B.H. Neuroprotective and neurodegenerative aspects of coffee and its active ingredients in view of scientific literature. Cureus 2020, 12, e9578.
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and adenosine a(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245.
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and neuroprotective effects of caffeine against Alzheimer’s and Parkinson’s disease: Insight into the role of nrf-2 and a2ar signaling. Antioxidants 2020, 9, 902.
- Faivre, E.; Coelho, J.E.; Zornbach, K.; Malik, E.; Baqi, Y.; Schneider, M.; Cellai, L.; Carvalho, K.; Sebda, S.; Figeac, M.; et al. Beneficial effect of a selective adenosine a2a receptor antagonist in the appswe/ps1de9 mouse model of Alzheimer’s disease. Front. Mol. Neurosci. 2018, 11, 235.
- Semwal, B.C.; Garabadu, D. Amyloid beta (1-42) downregulates adenosine-2b receptors in addition to mitochondrial impairment and cholinergic dysfunction in memory-sensitive mouse brain regions. J. Recept. Signal Transduct. Res. 2020, 40, 531–540.
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42.
- Becker, A.; Fassbender, K.; Oertel, W.H.; Unger, M.M. A punch in the gut—Intestinal inflammation links environmental factors to neurodegeneration in Parkinson’s disease. Park. Relat. Disord. 2019, 60, 43–45.
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 36.
- Tanaka, K.; Miyake, Y.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Intake of japanese and chinese teas reduces risk of Parkinson’s disease. Park. Relat. Disord. 2011, 17, 446–450.
- Ascherio, A.; Zhang, S.M.; Hernan, M.A.; Kawachi, I.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann. Neurol. 2001, 50, 56–63.
- Hellenbrand, W.; Seidler, A.; Robra, B.P.; Vieregge, P.; Oertel, W.H.; Joerg, J.; Nischan, P.; Schneider, E.; Ulm, G. Smoking and Parkinson’s disease: A case-control study in germany. Int. J. Epidemiol. 1997, 26, 328–339.
- Bakshi, R.; Macklin, E.A.; Hung, A.Y.; Hayes, M.T.; Hyman, B.T.; Wills, A.M.; Gomperts, S.N.; Growdon, J.H.; Ascherio, A.; Scherzer, C.R.; et al. Associations of lower caffeine intake and plasma urate levels with idiopathic Parkinson’s disease in the harvard biomarkers study. J. Park. Dis. 2020, 10, 505–510.
- Fujimaki, M.; Saiki, S.; Li, Y.; Kaga, N.; Taka, H.; Hatano, T.; Ishikawa, K.I.; Oji, Y.; Mori, A.; Okuzumi, A.; et al. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology 2018, 90, e404–e411.
- Kluss, J.H.; Mamais, A.; Cookson, M.R. Lrrk2 links genetic and sporadic Parkinson’s disease. Biochem. Soc. Trans. 2019, 47, 651–661.
- Crotty, G.F.; Maciuca, R.; Macklin, E.A.; Wang, J.; Montalban, M.; Davis, S.S.; Alkabsh, J.I.; Bakshi, R.; Chen, X.; Ascherio, A.; et al. Association of caffeine and related analytes with resistance to Parkinson disease among lrrk2 mutation carriers: A metabolomic study. Neurology 2020, 95, e3428–e3437.
- Ohmichi, T.; Kasai, T.; Kosaka, T.; Shikata, K.; Tatebe, H.; Ishii, R.; Shinomoto, M.; Mizuno, T.; Tokuda, T. Biomarker repurposing: Therapeutic drug monitoring of serum theophylline offers a potential diagnostic biomarker of Parkinson’s disease. PLoS ONE 2018, 13, e0201260.
- Hong, C.T.; Chan, L.; Bai, C.H. The effect of caffeine on the risk and progression of Parkinson’s disease: A meta-analysis. Nutrients 2020, 12, 1860.
- Maclagan, L.C.; Visanji, N.P.; Cheng, Y.; Tadrous, M.; Lacoste, A.M.B.; Kalia, L.V.; Bronskill, S.E.; Marras, C. Identifying drugs with disease-modifying potential in Parkinson’s disease using artificial intelligence and pharmacoepidemiology. Pharmacoepidemiol. Drug Saf. 2020, 29, 864–872.
- Chen, J.F.; Cunha, R.A. The belated us fda approval of the adenosine a2a receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal. 2020, 16, 167–174.
- Sako, W.; Murakami, N.; Motohama, K.; Izumi, Y.; Kaji, R. The effect of istradefylline for Parkinson’s disease: A meta-analysis. Sci. Rep. 2017, 7, 18018.
- Chen, J.F.; Xu, K.; Petzer, J.P.; Staal, R.; Xu, Y.H.; Beilstein, M.; Sonsalla, P.K.; Castagnoli, K.; Castagnoli, N., Jr.; Schwarzschild, M.A. Neuroprotection by caffeine and a(2a) adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 2001, 21, RC143.
- Luan, Y.; Ren, X.; Zheng, W.; Zeng, Z.; Guo, Y.; Hou, Z.; Guo, W.; Chen, X.; Li, F.; Chen, J.F. Chronic caffeine treatment protects against alpha-synucleinopathy by reestablishing autophagy activity in the mouse striatum. Front. Neurosci. 2018, 12, 301.
- Khadrawy, Y.A.; Salem, A.M.; El-Shamy, K.A.; Ahmed, E.K.; Fadl, N.N.; Hosny, E.N. Neuroprotective and therapeutic effect of caffeine on the rat model of Parkinson’s disease induced by rotenone. J. Diet. Suppl 2017, 14, 553–572.
- Pardo, M.; Paul, N.E.; Collins-Praino, L.E.; Salamone, J.D.; Correa, M. The non-selective adenosine antagonist theophylline reverses the effects of dopamine antagonism on tremor, motor activity and effort-based decision-making. Pharmacol. Biochem. Behav. 2020, 198, 173035.
- Rohilla, S.; Bansal, R.; Kachler, S.; Klotz, K.N. Synthesis, biological evaluation and molecular modelling studies of 1,3,7,8-tetrasubstituted xanthines as potent and selective a2a ar ligands with in vivo efficacy against animal model of Parkinson’s disease. Bioorg. Chem. 2019, 87, 601–612.
