Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Contrast-enhanced ultrasound (CEUS) is a noninvasive imaging technique that utilizes contrast agents consisting of microbubbles/nanobubbles of gas to enhance ultrasound imaging, allowing for assessment of the size, shape, texture, and vascularity of several organs. Contrast-enhanced ultrasound (CEUS) is an emerging technology in veterinary medicine involving the administration of intravenous contrast agents, and it is increasingly recognized for its high potential as a diagnostic imaging tool for small animals.
- dogs and cats
- spleen
- CEUS
1. Benign Diffuse Diseases
As previously stated, several studies in both human and veterinary medicine demonstrate the efficacy of using CEUS in differentiating between malignant and benign focal lesions of the spleen [41,58,69]. On the other hand, when it comes to the use of CEUS in the diagnosis of splenic diffuse alterations, there are few published works that emphasize its advantages over the conventional ultrasound. Examples are a splenomegaly (congestion, splenic hyperplasia/extra medullary hematopoiesis, and inflammatory splenomegaly), an accessory spleen, and an inhomogeneous spleen of unknown causes [70,71,72]. Table 1 summarizes CEUS findings in benign splenic lesions.
Table 1. Summary of CEUS findings in benign splenic lesions.
| Diagnosis of Malignancy | Diagnostic Procedure | n | Age (Mean) | Species | Sex | CEUS Findings | Contrast Medium | Stats. | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Nodular hyperplasia | Cytology * | 20 | * | D | * | Isoechoic wash-in, hypoechoic peak enhancement, and anechoic wash-out | Sulphur hexafluoride | CI 95% * | [73] |
| Nodular hyperplasia, | One or a combination of cytology and histopathology * | 7 | 10.1 yrs | D | M/F * | Variations in all phases *; no tortuous vessels seen | Sulfur hexafluoride | Sensitivity, specificity, and accuracy | [74] |
| extramedullary hematopoiesis, | 4 | ||||||||
| one reactive lymphoid tissue | 1 | ||||||||
| Nodular hyperplasia | Histopathology (6) and cytology (2) | 8 | 8.5 yrs | D | M (5), F (3) | Isoechoic vascular and hypoechoic parenchymal phases | Perflubutane | 2-tailed Fisher’s exact test, sensitivity, and specificity with 95% CI | [75] |
| Hematoma | Histopathology | 2 | 10.5 yrs | D | M (1), F (1) | Heteroechoic vascular and hypoechoic parenchymal | |||
| Extramedullary hematopoiesis | Histopathology | 2 | 9.5 yrs | D | M (2) | Isoechoic vascular and hetero-isoechoic parenchymal | |||
| Granuloma | Histopathology | 1 | 10 | D | M (1) | Isoechoic vascular and hypoechoic parenchymal | |||
| Hematoma | Histopathology | 5 | 10.6 yrs | D | M, F * | Not able to differentiate from hematomas | Perfluoropropane | Unpaired 2-tailed t-test | [76] |
| Hematoma with hyperplasia | 2 | ||||||||
| Nodular hyperplasia | Histopathology and cytology * | 6 | 10 yrs | D, C * | M, F * | Isoechoic in all phases without tortuous vessels | Sulfur hexafluoride | None reported | [77] |
| Extramedullary hematopoiesis | 2 | ||||||||
| Hematoma | 1 | Hypoechoic in all phases | |||||||
| Benign fibrous histiocytoma | 1 | Hyperechoic in all phases with dense vessels | |||||||
| Accessory spleen (splanunculus) | 1 | Isoechoic | |||||||
| Reactive hyperplasia | Histopathology and cytology * | 8 | 9.6 yrs | D | M, F * | Isoechoic and hyperechoic in wash-in and peak enhancement and variable in wash-out | Sulphur hexafluoride | Fisher’s exact test and odds ratios with 95% CI | [78] |
| Nodular hyperplasia | 10 | Variable in all phases | |||||||
| Extramedullary hematopoiesis | 6 | Generally isoechoic in all phases | |||||||
| Hematoma | 3 | Hypoechoic and hyperechoic in all phases | |||||||
| Leishmaniosis (normal spleen) | Cytology | 22 | 4.9 yrs | D | M, F * | Variable in all phases, depending on architecture of the spleen, and no difference in quantitative measurements were found | Sulphur hexafluoride | ANOVA | [79] |
* details unspecified by authors; yrs = years, D = dog, C = cat, M = male, F = female, CI = confidence interval.
Using CEUS, similar to the normal spleen, the enlarged spleen usually shows a homogeneous and marked enhancement of its texture, but this enhancement does not provide more diagnostic differential clues than the conventional ultrasonography [71].
Hyperplastic lymphoid tissue in benign hyperplastic diseases as reactive hyperplasia and splenic hematomas has been reported to be highly vascularized, and in a study with 60 dogs, these benign lesions presented a marked enhancement in CEUS. Nevertheless, this exam was of limited value and histology for confirmation of the benign nature is needed [78,79,80,81].
Leishmaniosis in dogs is known to be responsible for pathological changes in the spleen, namely splenomegaly and diffuse alterations of the eco-structure [82]. In leishmaniotic dogs that show small hypoechoic nodules through the spleen, marbled or moth-eaten lesions (also seen in extramedullary hematopoiesis and lymphoid hyperplasia hyperplasia) in gray scale ultrasound also showed an abnormal diffuse and persistent heterogeneous enhancement at 13, 33, and 60 s using CEUS [79].
CEUS was shown to be useful where there is doubt about the origin of a peri splenic mass (accessory spleen/splenunculis) or of tissue that has arisen post-splenectomy or post-trauma (splenosis) [58]. An accessory spleen has an incidence of around 16% in humans, and it is communally located at the splenic hilum and in the tail of the pancreas and presentes as nodules of variable size (from 1–4 cm) [72,83]. Normally, accessory spleens are easily identified with a conventional ultrasound, but large or atypically located spenunculi can cause diagnostic uncertainty, being misinterpreted as a pathological peritoneal nodule or enlarged lymph node [58,72]. CEUS can confirm that a mass represents ectopic splenic tissue, demonstrating an enhancement pattern typical of normal spleen, which is the persistent late phase enhancement, and differentiating the mass from other lesions such as pancreatic tail tumors, splenic hilar lymph nodes, adrenal lesions, ovarian masses, and metastatic deposits [58,71]. In veterinary medicine, accessory spleens are reported to be rare, but a study in 2010 [83] described the use of CEUS in four dogs where the masses were round to triangular, homogeneous, and hypoechoic, and located between the spleen, the stomach, and the pancreas, with results similar to the ones described above in humans.
In humans, CEUS is also helpful in demarcating and characterizing focal lesions in patients with an inhomogeneous splenic texture [71], as well as to demonstrate splenic involvement in some patients with the underlying diagnosis of malignant lymphoma and increase the diagnostic sensitivity in patients with granulomatous splenic involvement [80]. Nevertheless, in veterinary medicine, to our knowledge, there are no recent studies that demonstrate the clear advantages of using CEUS in the diagnosis or clarification of diffuse inhomogeneous changes in the spleen.
2. Malignant Splenic Lesions
Although malignant splenic lesions are relatively rare in human medicine [17,84,85,86], in recent years, the importance of correctly diagnosing malignant lesions [84,87,88,89,90], together with the risk of hemorrhage, immune system impairment, and sepsis associated with invasive procedures [84,89] has propelled research into the use of contrast-enhanced ultrasonography to differentiate between malignant and benign lesions in human medicine [69,88,91]. In veterinary medicine, neoplastic splenic lesions are much more common [92,93] and not easily differentiated from benign processes through normal imaging techniques [75,76,77]. Although long-held anecdotal beliefs, such as the presence of a cavitated splenic mass being indicative of neoplastic lesions, have proven to be unfounded, a diagnostic ultrasound continues to be a critical part of managing splenic lesions, as well as planning surgical approaches [94]. The fact that survival time after surgical intervention for those with benign lesions is significantly longer than those with neoplastic lesions [95,96] has profound implications on the decision-making process in the management of a case and whether euthanasia might be considered [97,98,99,100,101,102]. In an attempt to classify splenic lesions, ultrasound guided cytology is often the first approach [57,99,100,101], but this does not always provide a clear answer as to the nature of the lesion [77], and histopathological evaluation, considered the gold standard for the diagnosis of splenic lesions [57,96], must still be performed. This has resulted in the search for alternative forms of classifying splenic neoplastic lesions through the use of CEUS.
In human medicine, studies have shown that the use of CEUS does allow for the identification of malignant vs. benign splenic lesions, the former showing hypo-enhancement in the parenchymal phase independently of the enhancement in the arterial phase, with a faster wash-out rate compared to the surrounding normal splenic tissue [17,68,103,104,105,106]. In veterinary medicine, besides there being a greater range of histopathological changes in the spleen, they are more frequently found than in human medicine [91,92,93]. This has resulted in the study of a wider variety of neoplastic lesions and their CEUS characteristics. Table 2 summarizes CEUS findings in malignant splenic lesions.
Table 2. Summary of CEUS findings in malignant splenic lesions.
| Diagnosis of Malignancy | Diagnostic Procedure | n | Age (m) | Species | Sex | CEUS Findings | Contrast Medium | Stats. | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Focal histiocytic sarcomas | One or combination of cytology and histopathology * | 2 | 10.1 yrs | D | M/F | Hypoenhanced in parenchymal phase; tortuous vessels in all phases | Sulfur hexafluoride | Sensitivity, specificity, and accuracy | [74] |
| Hemagiosarocoma | 3 | ||||||||
| Hemagiosarocoma | Histopathology | 8 | 12 yrs | D | M (3), F (5) | Hypoenhanced in late vascular phase | Perflubutane | 2-tailed Fisher’s exact test, sensitivity, and specificity with 95% CI | [75] |
| Lymphoma | Cytology | 3 | 5 yrs | D | M (1), F (2) | ||||
| Histiocyticsarcoma | Histopathology | 2 | 9.5 yrs | D | M (1), F (1) | ||||
| Leiomyosarcoma | Histopathology | 1 | 14 yrs | D | F (1) | ||||
| Osteosarcoma | Histopathology | 1 | 12 yrs | D | F (1) | ||||
| Carcinoma | Histopathology | 1 | 11 yrs | D | F (1) | ||||
| Lymphosarcoma | Histopathology and cytology * | 7 | 10 yrs | D, C * | M, F * | Hypoechoic in wash-out phase (late vascular phase) | Sulfur hexafluoride | None reported | [77] |
| Hemangiosarcoma | 4 | ||||||||
| Malignant fibrous histiocytoma | 2 | ||||||||
| Undifferentiated sarcoma | 1 | ||||||||
| Histiocytic sarcoma | 1 | ||||||||
| liposarcoma | 1 | ||||||||
| Mast cell tumour | 1 | ||||||||
| Metastasis * | 1 | ||||||||
| Hemangiosarcoma | Histopathology | 11 | 10.6 yrs | D | M, F * | Not able to differentiate from hematomas | Perfluoropropane | Unpaired 2-tailed t-test | [76] |
| Hemangiosarcoma | Histopathology and cytology * | 10 | 9.6 yrs | D | M, F * | Hypoenhancement in wash-in and peak enhancement and wash-out phase | Sulphur hexafluoride | Fisher’s exact test and odds ratios with 95% CI | [78] |
| Malignant lymphoma | 6 | ||||||||
| Malignant histiocytosis | 5 | ||||||||
| Malignant fibrous histiocytoma | 1 | ||||||||
| Mesenchymal neoplasia | 3 | ||||||||
| Mast cell tumour | 2 | ||||||||
| Pancreatic adenocarcinoma metastasis | 1 | ||||||||
| Plasmocytoma | 1 |
* details unspecified by authors; yrs = years, D = dog, C = cat, M = male, F = female, CI = confidence interval.
Rossi et al. [77] studied 18 dogs and cats with 8 different types of malignant lesions and found that although the wash-in and peak phases showed some variations, all of them had hypo-enhanced wash-out phases. These results were confirmed in two other studies with 29 [78] and 16 [75] dogs, which included malignant lesions not observed in the original study: osteosarcoma, leiomyosarcoma [75], and plasmacytoma [78].
When looking at the characteristics of individual malignant lesions, malignant lymphomas were found to have the highest peak-intensity and AUC while also presenting the fastest wash-in and wash-out phases [56], while lympho-sarcomas showed only early wash-in and wash-out phases [77]. Hemangiosarcoma is the splenic neoplasia that has proven to be the hardest to diagnose definitively, presenting challenges even for histopathology. CEUS has proven unsuccessful in differentiating malignant hemangiosarcoma from a benign hematoma [76], and it has been suggested that the well-vascularized tissue present in areas of hemorrhage may also exist in areas of high cell proliferation, as in a hemangiosarcoma [104]. This maybe the reason why some hemangiosarcomas, in contrast with other malignant lesions, show hyperenhancement [76,78]. This could also present an explanation as to why aberrant vessels with corresponding high peak intensities have been found in hemangiosarcomas [74]. One study looking at five cases of malignant splenic lesions, of which there were hemangiosarcomas, found that although hypo-enhancement in the wash-out phase could not differentiate malignant from benign lesions, the presence of tortuous vessels feeding the lesion could [74]. This reinforces the possibility that differentiation between hematoma and hemangiosarcoma should focus on vascular CEUS patterns, a reflection of how the lesions are perfused [105], as opposed to contrast enhancement in any phase.
3. Guidelines for Interpreting the CEUS Findings of Splenic Masses
The introduction of CEUS has come to play an important role in the field of diagnostic imaging of splenic lesions, some of which are frequently identified during routine examination, especially in older animals. Though splenic pathology is often clinically silent, when compared to other abdominal organs, it can also be encountered by ultrasound routine exams, which usually provide valuable additional information about splenic abnormalities. Nonetheless, the characterization of focal splenic lesions by ultrasound can be quite difficult. Many lesions are often incidental findings and represent a diagnostic challenge. The conclusive diagnosis of various splenic pathologies is also difficult for the untrained eye and often can only be obtained by follow-up histopathological analysis. Overall, there is a lack of guidelines for CEUS splenic findings that may be used by veterinarians to make informed decisions and to guide them on their interpretation of CEUS findings in the splenic ultrasounds of cats and dogs.
Here, we combined the results obtained for malignant and benign splenic masses in CEUS findings for dogs and cats as a guide to help elaborate a list of differential diagnoses. In this way, lesions that may require diagnostics can be differentiated from those that can be safely dismissed or followed-up with regular ultrasound imaging. Figure 1 depicts the several interpretations that can be obtained when identifying splenic masses using CEUS, which are gathered in practical guidelines.
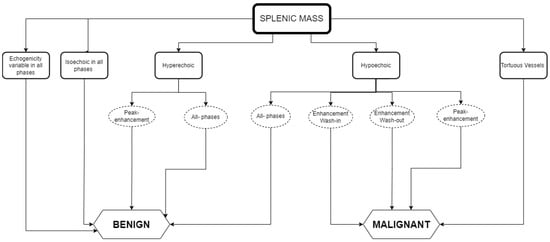
Figure 1. Guidelines for the CEUS data interpretation of splenic masses in cats and dogs: the relationship between the data obtained in CEUS and the type of splenic mass detected.
Whilst Figure 1 shows an interpretation guide for CEUS findings in suspicious splenic masses found in a CEUS exam. Figure 2 describes the type of diagnosis expected for benign and malignant splenic masses, according to the CEUS analysis results, using the data obtained in the literature described above in Table 1 and Table 2, and aimed at facilitating the decision-making process about whether to follow other diagnostic procedures such as cytology and histopathology.
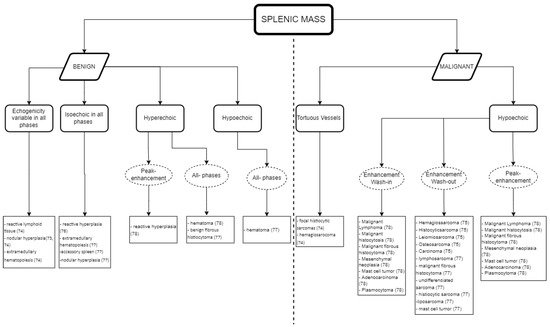
Figure 2. Guidelines for the CEUS data interpretation of splenic masses in cats and dogs: the expected diagnosis for benign and malignant splenic masses according to the data obtained in the CEUS examination.
As a practical approach, Figure 1 and Figure 2 can both be used for data interpretation, as well as for decision making regarding follow-up procedures. Although benign lesions are slightly more common than malignant lesions, an accurate diagnosis is often difficult and/or has a wider differential diagnosis. The diversity of the CEUS data obtained in spleen exams can frequently provide valuable additional information to narrow the differential diagnosis and, particularly, to pinpoint the lesions that are likely to be benign from those that may be malignant.
The summary figures provided can be used in clinical practice when faced with a splenic lesion on a B-mode ultrasound and when a CEUS scan is subsequently performed to clarify the nature of the lesion. Figure 1 can be followed after CEUS has classified the vascular pattern to establish the probability of the lesion being malignant or benign. Figure 2 can then be consulted to establish a list of possible differentials for the identified lesions. In this way, the decision-making process for recommending advanced and invasive diagnostics or adopting a wait and see approach can be rationalized.
This entry is adapted from the peer-reviewed paper 10.3390/ani12162104
This entry is offline, you can click here to edit this entry!
