Digital information technology is placing an increased cognitive load on our neurons. This enriched environment, provides ‘information-that-requires-action’, which acts through hormesis and activates the neuronal stress response. As a result, human neurons are under continual pressure to maintain themselves. Thus, repair resources must be allocated preferentially to the neuron, at the expense of the germline, through the bidirectional cross-talk between neuron vs germline. The result of this hormetic cognitive stress may be a reduction of age-related degeneration, which lasts indefinitely, with a corresponding reduction in reproduction.
- hormesis
- neuronal stress response
- neuron-germline communication
- neuron-germline conflict
1. Introduction
“This definition implies that, with the passage of time and for a variety of causative factors, humans are subjected to damage which is not properly repaired. As a consequence, there is degeneration and loss of utility at all levels (molecular, cellular, tissue, organismic, and societal) with a resulting failure of the normal function of a human. In other words, it is a chronologically-dependent erosion of our functions, which makes it increasingly difficult for us to manage and operate within a given, always-changing environment”.
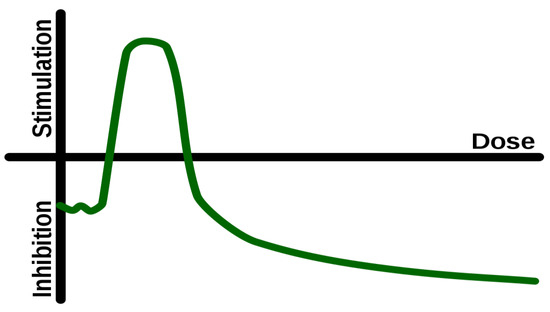
2. Hormesis
3. Autophagy and Hormesis
-
Exercise. This can enhance autophagy in liver, muscles, pancreas and adipose tissue, as well as in the brain [13].
-
Intermittent fasting (IF) is a nutritional hormetic stress. Alirezaei et al. [17] conducted a study to investigate the effects of food restriction and short-term fasting on autophagy. Their findings revealed that food restriction induces autophagy in mouse livers, challenging the conventional belief of the brain’s metabolic privilege. Moreover, their research suggests that sporadic fasting could be a cost-effective approach to promote a therapeutic neuronal response. In a separate study, Pietrocola et al. [18] emphasized the significance of autophagy in cancer treatment. They highlighted that impairment of autophagy reduces the effectiveness of chemotherapy and radiotherapy. These findings underscore the importance of understanding autophagic mechanisms to enhance cancer treatment strategies. Additionally, Kim and Lemasters [19] observed the occurrence of autophagy in liver cells during fasting, providing further insights into its role in cellular recycling. Their study demonstrated that liver cells form phagophores and autophagosomes, which encapsulate and capture mitochondria for recycling. This process leads to the breakdown of mitochondria and their contents, including DNA. In addition to physical stimuli, autophagy can be modulated by hormetins, i.e., substances that can induce health-beneficial physiological hormesis [20] and this is an appropriate opportunity to discuss some more details of hormetins.
4. Environmental Enrichment
5. Neuronal Stress Response
6. Digital Information, Cognition, and Neuronal Stress Response
“…evidence that video game interventions could be considered for the elderly for improving performance and cognitive function, especially general cognitive scores and processing speed. Games with better interactivity and visual stimulation have better curative effects…”.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12165433
References
- Kyriazis, M. Aging as “Time-Related Dysfunction”: A Perspective. Front. Med. 2020, 7, 371.
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97.
- Rattan, S. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78.
- Santoro, A.; Martucci, M.; Conte, M.; Capri, M.; Franceschi, C.; Salvioli, S. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res. Rev. 2020, 64, 101142.
- Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C.; Traverso, N. Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation. Antioxidants 2022, 11, 1613.
- Li, X.; Yang, T.; Sun, Z. Hormesis in Health and Chronic Diseases. Trends Endocrinol. Metab. 2019, 30, 944–958.
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7.
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly Generalizable and Beyond Laboratory. Trends Plant Sci. 2020, 25, 1076–1086.
- Pande, S.; Raisuddin, S. The Underexplored Dimensions of Nutritional Hormesis. Curr. Nutr. Rep. 2022, 11, 386–394.
- Demirovic, D.; Rattan, S.I. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp. Gerontol. 2013, 48, 94–98.
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? Aging. Mech. Dis. 2017, 3, 13.
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2019, 9, 790.
- He, C.; Sumpter, R.; Levine, B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 2012, 8, 1548–1551.
- Zhang, M.; Jiang, M.; Bi, Y.; Zhu, H.; Zhou, Z. Autophagy and Apoptosis act as partners to induce germ cell death after heat stress in mice. PLoS ONE 2012, 7, e41412.
- Penke, B.; Bogár, F.; Crul, T.; Sántha, M.; Tóth, M.E.; Vígh, L. Heat Shock Proteins and Autophagy Pathways in Neuroprotection: From Molecular Bases to Pharmacological Interventions. Int. J. Mol. Sci. 2018, 19, 325.
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337.
- Alirezaei, M.; Kemball, C.C.; Flynn, C.T.; Wood, M.R.; Whitton, J.; Kiosses, W.B. Short-term fasting induces profound neuronal autophagy. Autophagy 2010, 6, 702–710.
- Pietrocola, F.; Pol, J.; Vacchelli, E.; Rao, S.; Enot, D.P.; Baracco, E.E.; Levesque, S.; Castoldi, F.; Jacquelot, N.; Yamazaki, T.; et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016, 30, 147–160.
- Kim, I.; Lemasters, J.J. Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am. J. Physiol. Cell Physiol. 2011, 300, C308–C317.
- Rattan, S.I.S. Hormetins as drugs for healthy aging. In Anti-Aging Drugs: From Basic Research to Clinical Practice; Vaiserman, M., Ed.; The Royal Society of Chemistry: London, UK, 2017; pp. 170–180.
- Stavoe, A.K.H.; Holzbaur, E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019, 35, 477–500.
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct Target Ther. 2023, 8, 116.
- Martinelli, S.; Anderzhanova, E.A.; Bajaj, T.; Wiechmann, S.; Dethloff, F.; Weckmann, K.; Heinz, D.; Ebert, T.; Hartmann, J.; Geiger, T.M.; et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat. Commun. 2021, 12, 4643.
- Peker, N.; Gozuacik, D. Autophagy as a Cellular Stress Response Mechanism in the Nervous System. J. Mol. Biol. 2020, 432, 2560–2588.
- Cappucci, U.; Noro, F.; Casale, A.; Pimpinell, S. The Hsp70 chaperone is a major player in stress-induced transposable element activation. Proc. Natl. Acad. Sci. USA 2019, 116, 17943–17950.
- Kyriazis, M. Challenging Aging: The Anti-Senescence Effects of Hormesis, Environmental Enrichment, and Information Exposure; Frontiers in Aging Sciences; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; Volume 1, ISSN 2468-5933.
- Leon, M.; Woo, C. Environmental Enrichment and Successful Aging. Front. Behav. Neurosci. 2018, 12, 155.
- Colavitta, M.F.; Grasso, L.; Barrantes, F.J. Environmental Enrichment in Murine Models and Its Translation to Human Factors Improving Conditions in Alzheimer Disease. J. Prev. Alzheimers Dis. 2023, 10, 287–300.
- Birch, A.M.; Kelly, Á.M. Lifelong environmental enrichment in the absence of exercise protects the brain from age-related cognitive decline. Neuropharmacology 2019, 145, 59–74.
- Balietti, M.; Conti, F. Environmental enrichment and the aging brain: Is it time for standardization? Neurosci. Biobehav. Rev. 2022, 139, 104728.
- Schmidt, S.; Haase, M.; Best, L.; Groth, M.; Lindner, J.; Witte, O.W.; Kaleta, C.; Frahm, C. Restoring Age-Related Cognitive Decline through Environmental Enrichment: A Transcriptomic Approach. Cells 2022, 11, 3864.
- Barone, I.; Novelli, E.; Strettoi, E. Long-term preservation of cone photoreceptors and visual acuity in rd10 mutant mice exposed to continuous environmental enrichment. Mol. Vis. 2014, 20, 1545–1556.
- Levine, J.N.; Chen, H.; Gu, Y.; Cang, J. Environmental Enrichment Rescues Binocular Matching of Orientation Preference in the Mouse Visual Cortex. J. Neurosci. 2017, 37, 5822–5833.
- Gurfein, B.T.; Davidenko, O.; Premenko-Lanier, M.; Milush, J.M.; Acree, M.; Dallman, M.F.; Touma, C.; Palme, R.; York, V.A.; Fromentin, G.; et al. Environmental enrichment alters splenic immune cell and enhances secondary influenza vaccine responses in mice. Mol. Med. 2014, 20, 179–190.
- Vitalo, A.G.; Gorantla, S.; Fricchione, J.G.; Scichilone, J.M.; Camacho, J.; Niemi, S.M.; Denninger, J.W.; Benson, H.; Yarmush, M.L.; Levine, J.B. Environmental enrichment with nesting material accelerates wound healing in isolation-reared rats. Behav. Brain Res. 2012, 226, 606–612.
- Bice, B.D.; Stephens, M.R.; Georges, S.J.; Venancio, A.R.; Bermant, P.C.; Warncke, A.V.; Affolter, K.E.; Hidalgo, J.R.; Angus-Hill, M.L. Environmental Enrichment Induces Pericyte and IgA-Dependent Wound Repair and Lifespan Extension in a Colon Tumor Model. Cell Rep. 2017, 19, 760–773.
- Dorfman, D.; Aranda, M.L.; González Fleitas, M.F.; Chianelli, M.S.; Fernandez, D.C.; Sande, P.; Rosenstein, R.E. Environmental enrichment protects the retina from early diabetic damage in adult rats. PLoS ONE 2014, 9, e101829.
- Clark, B.C.; Mahato, N.K.; Nakazawa, M.; Law, T.D.; Thomas, J.S. The power of the mind: The cortex as a critical determinant of muscle strength/weakness. J. Neurophysiol. 2014, 112, 3219–3226.
- Zhao, Y.; Chen, K.; Shen, X. Environmental enrichment attenuated sevoflurane-induced neurotoxicity through the PPAR-γ signaling pathway. Biomed. Res. Int. 2015, 2015, 107149.
- Scarola, S.; Bardi, M. Environmental enrichment modulates inflammation during development in long-evans rats (Rattus norvegicus). Dev. Psychobiol. 2021, 63, 183–191.
- Matur, E.; Akyaz, İ.; Eraslan, E.; Ergul Ekiz, E.; Eseceli, H.; Keten, M.; Metiner, K.; Aktaran Bala, D. The effects of environmental enrichment and transport stress on the weights of lymphoid organs, cell-mediated immune response, heterophil functions and antibody production in laying hens. Anim. Sci. J. 2016, 87, 284–292.
- Wolinsky, F.; Unverzagt, F.W.; Smith, D.M.; Jones, R.; Wright, E.; Tennstedt, S.L. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J. Gerontol. B 2006, 61, S281–S287.
- Rebok, G.W.; Ball, K.; Guey, L.T.; Jones, R.N.; Kim, H.Y.; King, J.W.; Marsiske, M.; Morris, J.N.; Tennstedt, S.L.; Unverzagt, F.W.; et al. ACTIVE Study Group. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 2014, 62, 16–24.
- Joëls, M.; Karst, H.; Sarabdjitsingh, R.A. The stressed brain of humans and rodents. Acta Physiol. 2018, 223, e13066.
- Korneeva, N.L. Integrated Stress Response in Neuronal Pathology and in Health. Biochemistry 2022, 87 (Suppl. S1), S111–S127.
- Farley, M.M.; Watkins, T.A. Intrinsic Neuronal Stress Response Pathways in Injury and Disease. Annu. Rev. Pathol. 2018, 13, 93–116.
- Kim, K.W.; Jin, Y. Neuronal responses to stress and injury in C. elegans. FEBS Lett. 2015, 589, 1644–1652.
- Schulz, A.; Sekine, Y.; Oyeyemi, M.J.; Abrams, A.J.; Basavaraju, M.; Han, S.M.; Groth, M.; Morrison, H.; Strittmatter, S.M.; Hammarlund, M. The stress-responsive gene GDPGP1/mcp-1 regulates neuronal glycogen metabolism and survival. J. Cell Biol. 2020, 219, e201807127.
- Freeland, K.; Boxer, L.M.; Latchman, D.S. The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res. Mol. Brain Res. 2001, 92, 98–106.
- Korte, M. The impact of the digital revolution on human brain and behavior: Where do we stand? Dialogues Clin. Neurosci. 2020, 22, 101–111.
- Loh, K.K.; Kanai, R. How Has the Internet Reshaped Human Cognition? Neuroscientist 2016, 22, 506–520.
- Menghini, F.; van Rijsbergen, N.; Treves, A. Modelling adaptation aftereffects in associative memory. Neurocomputing 2007, 70, 2000–2004.
- Small, G.W.; Lee, J.; Kaufman, A.; Jalil, J.; Siddarth, P.; Gaddipati, H.; Moody, T.D.; Bookheimer, S.Y. Brain health consequences of digital technology use. Dialogues Clin. Neurosci. 2020, 22, 179–187.
- Advanced Cognitive Training in Vital Elderly—ACTIVE—Study. IU School of Medicine. 16 November 2017. Available online: https://medicine.iu.edu/news/2017/11/brain-exercise-dementia-prevention (accessed on 2 June 2023).
- Abd-Alrazaq, A.; Abuelezz, I.; AlSaad, R.; Al-Jafar, E.; Ahmed, A.; Aziz, S.; Nashwan, A.; Sheikh, J. Serious Games for Learning Among Older Adults with Cognitive Impairment: Systematic Review and Meta-analysis. J. Med. Internet Res. 2023, 25, e43607.
- Abd-Alrazaq, A.; Alhuwail, D.; Ahmed, A.; Househ, M. Effectiveness of Serious Games for Improving Executive Functions Among Older Adults with Cognitive Impairment: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e36123.
- Yang, C.; Han, X.; Jin, M.; Xu, J.; Wang, Y.; Zhang, Y.; Xu, C.; Zhang, Y.; Jin, E.; Piao, C. The Effect of Video Game-Based Interventions on Performance and Cognitive Function in Older Adults: Bayesian Network Meta-analysis. JMIR Serious Games 2021, 9, e27058.
- Clemenson, G.D.; Stark, S.M.; Rutledge, S.M.; Stark, C.E.L. Enriching hippocampal memory function in older adults through video games. Behav. Brain Res. 2020, 390, 112667.
- Ramnath, U.; Rauch, L.; Lambert, E.V.; Kolbe-Alexander, T. Efficacy of interactive video gaming in older adults with memory complaints: A cluster-randomized exercise intervention. PLoS ONE 2021, 16, e0252016.
- Chen, H.Y.; Jolly, C.; Bublys, K.; Immler, S. Trade-off between somatic and germline repair in a vertebrate supports the expensive germ line hypothesis. Proc. Natl. Acad. Sci. USA 2020, 117, 8973–8979.
