Autoimmune hemolytic anemia (AIHA) is a rare, very heterogeneous, and sometimes life-threatening acquired hematologic disease characterized by increased red blood cell (RBC) destruction by autoantibodies (autoAbs), either with or without complement involvement. AIHA can be primary (idiopathic) but is more often secondary, triggered by infections or drug use or as a part of other diseases. As the location of origin of autoAbs and the location of autoAb-mediated RBC clearance, as well as the location of extramedullary hematopoiesis, the spleen is crucially involved in all the steps of AIHA pathobiology. Splenectomy, which was the established second-line therapeutic option in corticosteroid-resistant AIHA patients for decades, has become less common due to increasing knowledge of immunopathogenesis and the introduction of targeted therapy.
1. Introduction
Autoimmune hemolytic anemia (AIHA) is an acquired autoimmune disease characterized by the increased destruction of autologous red blood cells (RBCs) due to the presence of immunoglobulin (Ig)G, IgM, IgA, or complements (usually C3d) bound to RBC membrane antigens [
1,
2,
3]. Although this rare, very heterogeneous, and sometimes life-threatening condition occurs across all age groups, with a reported annual incidence of 1–3 per 100,000 persons, the incidence of chronic and relapsing cases increases with age (i.e., over the age of 40) [
2].
Shortened RBC survival due to hemolysis clinically manifests in symptoms which include weakness, dyspnea, jaundice, acrocyanosis or Raynaud phenomena, and splenomegaly, and laboratory tests reveal normocytic/macrocytic anemia, reticulocytosis, and elevated indirect (unconjugated) bilirubin and lactate dehydrogenase with reduced or fully consumed haptoglobin. The spherocytosis, agglutination, and polychromasia of RBCs can be seen in a peripheral blood smear. Hemoglobinuria and the presence of urinary hemosiderin indicate the onset of extravascular hemolysis [
3].
The severity of AIHA is determined by the type and affinity of autoantibodies (autoAbs), which reduce RBC lifespan, and by the capacity of bone marrow to compensate for hemolysis. A diagnosis of hemolytic anemia is issued when RBC survival drops below 100 days [
4]. The spleen plays a major role in the pathobiology of AIHA, both in the synthesis of autoAbs and in the immune destruction of RBCs.
2. The Role of the Spleen and Bone Marrow in the Immunological Background of AIHA
2.1. Spleen as the Place of Origin of Autoantibodies in AIHA
Hitherto, the pathogenesis of AIHA has not been fully understood. Today, it is believed that, as in autoimmune and lymphoproliferative diseases, the breakdown of immunological central and peripheral tolerance, T- and B-cell dysregulation, and a shift towards T-helper 2 (Th2) and Th17 phenotype play major roles in the generation of autoAbs directed against RBCs.
The spleen is the largest secondary lymphoid organ and plays an important role in host defense but also in autoimmunity. The spleen is divided by function and structure into red and white pulp, with a perifollicular (marginal) zone in between. The primary immunological region of the spleen comprises white pulp, which occupies less than a quarter of splenic tissue. Unlike the lymph nodes, the spleen lacks afferent lymphatic vessels, and therefore, all cells and antigens enter the spleen via the blood [
15]. The secretion of Abs by lymphoid follicle germinal center (GC) plasma cells (PCs) that have lifespans of 2–3 days is tightly regulated by negative feedback interactions with follicular Th cells (Tfh). Tfh cells produce IL-21, a protein critical for the processes of affinity maturation, GC longevity and function, and B-lymphocyte terminal differentiation [
16]. Conventional PCs are designated to participate in bone marrow homing. Embedded in bone marrow niches, PCs may survive as long-lived PCs (LLPCs), persisting for up to the lifetime of the hosts in the absence of repeated antigen stimulation, together with memory CD4+ and memory CD8+ T cells [
17,
18].
The association between autoimmune disorders and neoplastic diseases, especially lymphoid neoplasms of B-cell origin (chronic lymphocytic leukemia(CLL), B-cell non-Hodgkin’s lymphoma, and Hodgkin’s lymphoma), was noted a long time ago. Autoimmune diseases, predominantly rheumatological (systemic lupus erythematosus, rheumatoid arthritis, and ankylosing spondylitis), endocrinological (type-1 insulin-dependent diabetes, Hashimoto thyroiditis, and Graves’ disease), neurological (multiple sclerosis and myasthenia gravis), and dermatological (pemphigus vulgaris) ones, have been linked with various autoimmune cytopenias (anemia, thrombocytopenia, neutropenia, pure red cell aplasia) and also with lymphoproliferative diseases and solid cancer. They share a common underlying etiology and are caused by the impaired self-tolerance of the immune system [
14,
23,
24,
25,
26]. AIHA may occur synchronously with lymphoma, may precede or follow its diagnosis for several years, and may even be related to antilymphoma therapy [
24].
The relationship between AIHA and congenital conditions such as common variable immunodeficiency/hyper IgM syndrome/autoimmune lymphoproliferative syndrome/Kabuki syndrome suggest that genetic background is an important factor regarding AIHA onset. Recurrent somatic mutations of
KMT2D and mono-allelic
CARD11 were demonstrated in patients with CAD. The loss of KMT2D function is related to the stimulation of the auto-reactive
IGHV4-34-encoded immunoglobulin receptor, disturbed class switch recombination, and enhanced B-cell proliferation and survival. Mono-allelic
CARD11 mutations result in B-cell proliferation and auto-antibody production. Similarly, oncogenic bi-allelic
CARD11 mutations that result in constitutive nuclear factor (NF)-κB activation were demonstrated in a subset of patients with diffuse large B-cell lymphoma [
16].
Conversely, the origins of autoAbs in drug-induced AIHA (DIHA) are well established. More than 160 drugs are suspected of inducing AIHA, the most commonly reported being antibiotics (penicillins, cephalosporins, and cotrimoxazole), antimycotics (fluconazole and amphotericin B), diclofenac, ibuprofen and other non-steroidal anti-inflammatory drugs, immunosuppressive (azathioprine) and antineoplastic drugs (both conventional ones like fludarabine, chlorambucil, and bendamustine, and novel options, such as immune checkpoint inhibitors), cardiovascular drugs (methyldopa, furosemide, and enalapril), and many others, including corticosteroids [
2,
29,
30]. It is worth mentioning that some vaccines, including the mRNA-COVID 19 vaccine, have also been implicated as causes of the new onset or relapse of AIHA and Evans syndrome [
31]. Although rare, DIHA encompasses about 10% of all AIHA cases [
32].
2.2. Immune Clearance of Autoantibodies by Spleen Macrophages
Although an adaptive immune response to foreign antigens is initiated in the white pulp, immune effector function takes place and expands in spleen red pulp where neutrophils, monocytes, dendritic cells (DCs), gamma delta (γδ) T cells, and macrophages reside [
17]. The most abundant Ig isotype in human serum and the predominant anti-RBC autoAb in wAIHA is IgG. IgG is composed of four subtypes whose constant regions differ in terms of the hinges and CH2 domains that are involved in binding to IgG-Fc receptors (FcγR) and C1q. The binding of IgG to FcγR on effector cells (macrophages, CD8+ T cells, and natural killer (NK) cells) triggers phagocytosis and antibody-dependent cell-mediated cytotoxicity (ADCC). In physiological conditions, IgG1, which is the most abundant IgG subclass, and IgG3 interact efficiently with most FcγR, while IgG2 and IgG4 show reduced affinity to a number of FcγR [
34].
Extravascular FcγR-mediated hemolysis takes place in the spleen and lymphoid organs. Spleen macrophages regulate RBC turnover in physiological and pathological circumstances. During a normal lifespan, RBCs encounter detrimental changes in plasma cell membrane and become less deformable. Senescent RBCs are too rigid to pass through the inter-endothelial slits of the spleen red pulp, and those trapped RBCs are phagocytized by macrophages located in the cords of the red pulp. Every day, approximately 1% of aged and irreversibly damaged RBCs are removed from circulation [
36].
The most abundant integral membrane protein in RBCs is Band 3 (SLC4A1), with more than a million copies per cell. Band 3 is associated with a number of other membrane proteins including the Rh complex, glycophorins, and CD47. At the inner RBC membrane, Band 3 is attached to the cytoskeleton through interaction with ankyrin, while its carboxyl terminus is associated with carbonic anhydrase. Consequently, Band 3 has numerous fundamental functions in maintaining RBC integrity—from controlling RBC shape and deformability, through regulating CO
2 transport, to mediating phagocytosis in the spleen. Namely, Band 3 exposes neoantigens that are recognized by naturally occurring Abs (Nabs) and cleared by macrophages. Conversely, CD47 represents one of the “do not eat me” signals, and binding to its receptor, signal-regulatory protein alpha (SIRPα), on macrophages suppresses phagocytosis by inhibiting the inside-out activation of integrin signaling [
35]. In wAIHA, as well as in immune thrombocytopenia patients, CD47 is expressed at normal levels [
38]. Spleen macrophages are not just responsible for clearing aged and damaged RBCs but also for repairing RBCs, as seen in the removal of inclusion bodies from circulating RBCs via spleen-facilitated vesiculation [
39].
Splenic macrophages possess three types of FCγR receptors for the IgG heavy chain that activates phagocytosis: FcγRI (CD64), FcγRIIA (CD32a), and FcγRIII (CD16). Phagocytosed RBCs are targeted to phagolysosomes. FcγRI has the highest affinity for IgG molecules, and its activation induces signaling pathways. This includes phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAP), which are responsible for efficient erythrophagocytosis and a minimal release of harmful free hemoglobin into circulation. It is of clinical interest that spleen macrophages also express an inhibitory FcγRIIB and that its activation may explain the inefficiency of intravenous Ig (IVIG) in wAIHA as their therapeutic activity is mediated through binding to FcγRIIB [
35].
Besides phagocytosis, spleen macrophages can only remove the IgG-coated portion of RBC membranes, resulting in a change in shape and RBCs becoming deformable. The formed rigid microspherocytes are retained in spleen red pulp sinusoids and cleared, as previously explained. Spleen CD8+ T cells and NK cells that also express FcγR are responsible for the ADCC destruction of opsonized RBCs [
1,
2,
5,
23,
35].
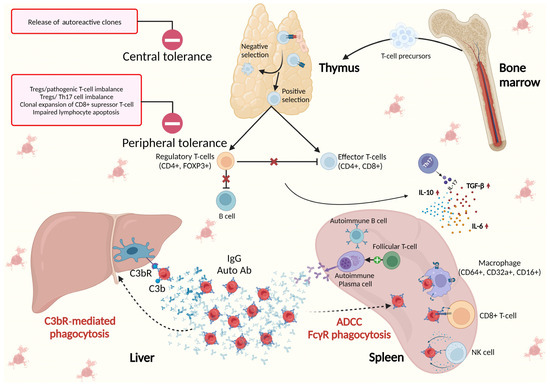
The pivotal role of the spleen in the immunological background of AIHA is illustrated in Figure 2.
Figure 2. The spleen in the immunological background of AIHA. Central tolerance takes place in primary lymphoid organs (bone marrow for B-cells and thymus for T-cells) and is responsible for identifying immature self-reactive lymphocytes, i.e., T- or B-cell clones that possess receptors, to identify self-antigens with high affinity. These autoreactive clones, upon binding with self-antigens, undergo deletion or apoptosis (negative selection) or may, in the case of B-cells, change their specificity or, in the case of T cells, develop regulatory tolerance (Treg). Peripheral tolerance takes place in the germinal centers (GCs) of lymphoid follicles in secondary lymphoid organs (the spleen, lymph nodes, and mucosal lymphoid tissues) where foreign antigens are normally encountered. The importance of peripheral tolerance in preventing autoimmunity is in maintaining unresponsiveness to self-antigens that are expressed in peripheral tissues and not in primary lymphoid organs, and in preserving tolerance to self-antigens that are expressed in adult life after the production of mature lymphocytes. Peripheral tolerance is driven by specific CD4+FOXP3+ Tregs, and the imbalance between Tregs and pathogenic effector/memory CD+ T cells, as well as Tregs and Th17, together with increased clonal expansion of CD8+ suppressor T cells and impaired lymphocyte apoptosis, lead to the loss of control over T-cell activation and autoimmunity [
14,
23,
40]. Th17 cells play crucial roles in the immune response against microorganisms and in autoimmunity and facilitate the development and progression of various cancers. They are responsible for the secretion of proinflammatory cytokine interleukin-17 (IL-17), which promotes and characterizes humoral autoimmune response, together with observed increased levels of IL-6, IL-10, and transforming growth factor (TGF)-β and reduced tumor necrosis factor (TNF)-α [
15,
26,
40]. In contrast to normal plasma cells (PCs), autoimmune plasma cells accumulate in the spleen, establishing positive feedback with follicular T-helper cells (Tfh). Opsonized erythrocytes undergo elimination in the spleen through FcγR-mediated phagocytosis and antibody-dependent cellular cytotoxicity (ADCC), as well as in the liver via C3bR phagocytosis.
3. The Place of Splenectomy in the Era of Novel AIHA Treatment
The enlargement of spleen in AIHA is the consequence of Ab production, the immune destruction of RBC, and extramedullary hematopoiesis, crucial factors in IHA pathobiology. The surgical removal of the spleen was recognized and widely accepted as an effective therapeutic option in corticosteroid-resistant AIHA cases a long time ago. In a study by Allgoold and Chaplin on 44 AIHA patients published in 1967, an early response was obtained after splenectomy in 68% of corticosteroid-resistant patients, and 44% were able to maintain remission without supplementary steroid therapy after more than one year post-splenectomy. On the other side, the long-range mortality rate in this cohort was 40%, and the most common cause of death was pulmonary embolism [
95]. Excellent efficacy was reported two decades later in a study on 52 splenectomized AIHA patients [
96], with a reported sustained complete response rate of 64% after a mean follow-up period of 33 months, minimal morbidity, and an absence of mortality. Splenectomy was confirmed to be less efficient in secondary AIHA. A retrospective review of 30 patients with AIHA who underwent splenectomy revealed significantly inferior outcomes for AIHA patients with associated diseases (the most prevalent were B-cell malignancies and rheumatologic disorders), with an overall response rate of 56% and complete remission achieved in only three out of sixteen (19%) cases, while all patients with idiopathic AIHA demonstrated a response to splenectomy that was complete in nine out of eleven (82%) instances. Of interest is the fact that the reported median time from AIHA diagnosis to splenectomy in both groups was very short, at only 3.2 months for patients with secondary AIHA, and that seven patients within this group had associated thrombocytopenia, indicating Evans syndrome, a condition that may partly explain their worse outcome [
97]. The irradiation of the spleen was successfully performed in several patients with associated lymphoproliferative diseases and high perioperative risk. As splenic irradiation in this setting can be considered experimental, a total irradiation dose is not established for performing these procedures and in published cases varies from 8 Gy to 20 Gy. It is assumed that a higher radiation dose may induce a “functional” splenectomy [
98].
In the years that followed, although still prior to the introduction of novel therapy in the treatment of AIHA and underling diseases, the reported median time from AIHA diagnosis to splenectomy extended and the laparoscopic procedure prevailed over the open-surgery technique [
99,
100]. Due to the rarity of AIHA, patients with secondary AIHA were included in most published studies. In a case series of nine patients with symptomatic and uncontrolled AIHA diagnosed concurrently with CLL, splenectomy was performed after the failure of both CLL-targeted treatment with alkylating agents and/or purine analogues and AIHA targeted treatment with IVIG and/or corticosteroids. A median time to splenectomy of 62 months was reported, with patients’ average age at the time of splenectomy being 62 years. Seven of the nine patients (78%) achieved early complete response, and six (67%) retained remission from AIHA within a mean follow-up of 24 months after splenectomy. All patients underwent splenectomy via the laparoscopic technique and seven of the nine patients (78%) experienced no immediate postoperative complications [
100]. Higher response rates were achieved in patients with primary AIHA. Long-term postoperative follow-up evaluations were obtained in three studies on the efficiency of laparascopic splenectomy performed in the context of malignant and non-malignant hematologic conditions, including primary AIHA [
101,
102,
103].
According to the current guidelines, splenectomy or partial splenic embolization are still recommended in the emergency management of transfusion-dependent life-threatening wAIHA that is unresponsive to prednisolone. This should be combined with other supportive therapies (erythropoietin stimulating agents (ESA) and/or plasma exchange and/or IVIG) and active immunosuppression [
5,
55].
The loss of crucial functions of splenic macrophages in eliminating encapsulated bacteria and prolonged antecedent or concomitant immunosuppression renders splenectomized AIHA patients highly susceptible to bacterial infections, with a reported rate of 6–7% [
112]. Among the 4756 AIHA patients identified using the California Discharge Dataset 1991–2014, the cumulative incidence of sepsis was 4.3% in those who had never undergone a splenectomy, compared to rate of 6.7% who had undergone a splenectomy. For the latter group, the rate of sepsis only increased in the late postoperative period [
113]. In addition to postoperative antibiotic prophylaxis, vaccination with polysaccharide and conjugate vaccines against Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis should be performed at least 14 days before a scheduled splenectomy, or after the 14th postoperative day after an urgent splenectomy [
114,
115]. The use of live or live, attenuated vaccines in patients receiving >10 mg/day of prednisone or a cumulative dose of >700 mg over three months should be avoided, and the Food and Drug Agency recommended deferring procedures until at least one month after steroid discontinuation [
116]. This is a need that cannot always be met in the case of refractory AIHA patients.
Thromboprophylaxis is also indicated in splenectomized AIHA patients in order to minimize the risk of venous thrombosis. It has long been recognized that AIHA has a prothrombotic state, per se [
117], and thromboprophylaxis is advisable and recommended for all hospitalized patients admitted for wAIHA, as well as for outpatients with marked hemolysis [
5,
32,
54]. The risk of venous thrombosis is further increased in splenectomized AIHA patients, leading to long-term morbidity and disability [
113,
118,
119].
Splenectomy is not recommended to treat complement-mediated intravascular hemolysis, nor is it recommended for complement-mediated extravascular hemolysis where CD3b-opsonized RBCs are destroyed via liver macrophages that carry receptors for C3b fragments [
51]. This is despite the fact that several case reports demonstrating the successful management of refractory CAD with splenectomy have been published, especially in recent years [
120].
4. Conclusions
The spleen is the location of origin of autoAb and the location of antibody-mediated RBC phagocytosis in AIHA, as well as the location of extramedullary hematopoiesis. Splenectomy was the therapeutic option in corticosteroid-resistant AIHA patients for decades. In the era of modern therapy, splenectomy is scarcely performed, as it is either employed as a third or subsequent line of treatment or is recommended as a rescue management technique for transfusion-dependent, life-threatening wAIHA. The reported rate of complications following splenectomy has gradually decreased over time due to less invasive laparascopic techniques, vaccination against encapsulated bacteria, and thromboprophylaxis. Today, splenectomy is not associated with increased mortality. When taking into account this and the real-world evidence of the limited wide availability of rituximab and other costly targeted therapies inspired by clinical trials in most and not only developing countries, laparascopic splenectomy holds its place as a safe and good therapeutic option in the selected pools of AIHA patients.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13182891