Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The concept of intelligent/smart insulins is straightforward, and it can be summed up as “from complexity to simplicity.” In essence, it is a question of creating an insulin capable of self-regulating according to changes in blood sugar, so-called “glucose responsive insulin” (GRI), eliminating the need to consider all the variables involved in dose calculation (calories, carbohydrates, exercise, time, etc.).
- intelligent insulin
- glucose-responsive insulin
- concanavalin A (ConA)
- phenylboronic acid
- glucose oxidase
1. Introduction
GRI may be formulated as polymer-based systems, wherein insulin is encapsulated within a glucose-responsive polymeric matrix-based vesicle or hydrogel [1] or as a molecular GRI analogue system, which involves the introduction of a glucose-sensitive motif to the insulin molecule; in either case, its formulation confers glucose-responsive changes to insulin bioavailability or hormonal activity [2][3].
Polymer-based technologies employ the sequestration of insulin within a matrix suitable for subcutaneous injections. Stimuli-responsive polymers (hydrogels) can convert environmental stimuli (i.e., temperature, pH, ionic strength, glucose concentration) into the signal to trigger the change in physical properties of the polymers themselves [4][5]. The matrix, in principle, senses the glucose concentration and releases a proportional amount of insulin.
To date, the principal mechanisms underlying artificial smart GRI delivery systems are (Figure 1):
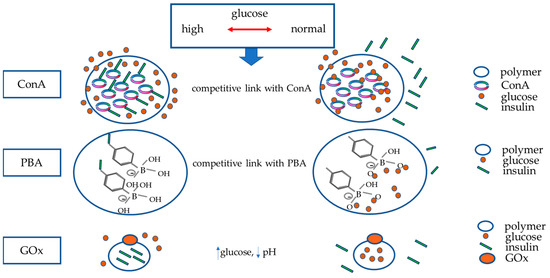
Figure 1. Principal mechanisms underlying artificial smart GRI delivery systems. The figure schematically represents the three mechanisms currently studied to build glucose-responsive insulin systems (nanoparticles or vesicles able to disassemble by swelling or degradation in response to increased glucose): Concanavalin A (ConA) and phenylboronic acid (PBA) release insulin once they are competitively bound by glucose. As blood sugar decreases, they will bind to insulin again. Polymers in which glucose oxidase (GOx) has been inserted will release insulin in response to the decrease in pH in the case of hyperglycaemia; GOx enzymatically converts glucose to gluconic acid, producing a drop in pH. This mechanism is also reversible when euglycemia is restored.
-
Glucose-binding proteins, a class that includes lectins, like concanavalin A (ConA);
-
Phenylboronic acid (PBA);
-
Glucose oxidase (GOx).
2. Glucose-Binding Proteins
In 1979, a stable, biologically active glycosylated insulin derivative that was complementary to the major combining site of concanavalin A (ConA) was synthesized as a pioneering GRI system. ConA is a protein of the lectin family that can reversibly and specifically bind to glucose and mannose with a high affinity [6].
Hormone release was proportional to the quantity of glucose present [7], and the complex was designed to sequester insulin in the subcutaneous space during normoglycaemia and release the hormone during hyperglycaemia via competition with ambient glucose molecules.
Responsive hydrogel(s) were formulated from glucosyloxyethy1 methacrylate (GEMA), N,N′-methylene-bis-acrylamide (MBAAm) [8][9] and porous poly(hydroxyethyl methacrylate) (PHEMA) [10]. The rise of glucose concentration led to a decrease of gel density, with an insulin release.
It is also possible to conjugate insulin to various glucose-like molecules: the combination of glucose-modified insulin and the glucose-binding lectin, ConA, leads to a dissociation/reassociation under increased/decreased glucose concentrations [6][11].
ConA’s immunogenicity and mitogenicity limit the clinical translation [12] of these approaches, and a major challenge is that the self-regulation of insulin release from the GRI-ConA system in response to different glucose levels will not be always reproducible [6].
3. Phenylboronic Acid (PBA)
Phenylboronic acid (PBA) is a diol-binding element that exhibits a strong affinity for sugars, such as D-glucose [13]. The competitive binding of the hydroxyl groups of D-glucose to borate ion increases the swelling of the glucose-PBA complex. One major concern for practical application is that the pH dependence of PBA ionization at physiological conditions is not guaranteed. Various approaches have been conducted to use PBA in GRI systems.
As GRI, it was demonstrated that a modified insulin, carrying a PBA and a polyol group attached to the LysB29 sidechain, forms a high molecular weight multimeric complex that dissociates as D-glucose concentration rises [2]. A similar mechanism was applied to insulin degludec [14] and detemir [15]. Insulin detemir was modified through the incorporation of an aliphatic domain to facilitate hydrophobic interactions and a PBA for glucose sensing. This GRI affords long-lasting and glucose-responsive activity in the diabetic mouse, providing a potentially improved therapeutic strategy for diabetes management [15].
PBA’s reversible binding to glucose provides a potential mechanism for designing insulin formulations that respond to changes in blood glucose concentrations, so various approaches (glucose-triggered swelling, dissociation, competitive replacement) have been conducted to use PBA in GRI systems [4]. Several matrices (gels, micelles, capsules) have been synthesized with the use of PBA. Thanks to PBA’s ability to switch from a hydrophobic form to a hydrophilic form, insulin may or may not be released in response to glucose levels [4].
Various hydrogels were prepared with in vitro and, in some cases, in vivo results. Matsumoto described a catheter-combined device using PBA gel that was suitably scaled for mouse model experiments and demonstrated that it could serve as an “artificial pancreas” in diabetic mice [16]. The effect of the device on glucose metabolism in streptozotocin (STZ)-induced type 1 diabetes mice, was studied: seven days after implantation of the recombinant human insulin-loaded device, there was a marked reduction in glycaemia and water intake, with at least 3-week durability, potentially dependent of the volume of the reservoir [16].
4. Glucose Oxidase (GOx)
Contrary to ConA and PBA-based systems, glucose oxidase (GOx)-coupled systems do not directly bind or form a complex with glucose molecules to exhibit sensitivity. Utilizing GOx enzymes, circulating glucose and oxygen can be initially oxidized into gluconic acid and hydrogen peroxide end-products. The gluconic acid is used as a trigger for responsiveness when coupled with pH-responsive materials in the second step of this process. Thus, entrapping or cross-linking GOx with pH-responsive polymers can cause rapid-phase swelling/deswelling in response to environmental glucose.
Glucose oxidase (β-D-glucose:oxygen 1-oxidoreductase, EC 1.1.3.4) is a flavoprotein that catalyses the oxidation of β-D-glucose at its first hydroxyl group, utilizing molecular oxygen as the electron acceptor, to produce D-glucono-delta-lactone (gluconic acid) and hydrogen peroxide (H2O2) [17]. The glucose-oxidation reaction catalysed by GOx changes the physiological environment, modifying pH, H2O2 concentration, and O2 levels.
A polymer with tertiary amino groups is protonated by a decrease in the pH value of a medium, and the hydrophilicity of the polymer increases. A glucose-responsive polymer membrane can be obtained by combining a poly amine membrane and GOx immobilized membrane since the pH value of the medium will decrease by the formation of gluconic acid produced from the reaction of GOx with glucose. A GRI system could be prepared from N, N-diethylaminoethyl methacrylate [18], poly methacrylic acid-g-ethylene glycol [19], acid-responsive chitosan matrix, or GOx/catalase nanocapsules [20]. A glucose-responsive microgel composed of an acid-responsive chitosan matrix, GOx/catalase (CAT) nanocapsules, and recombinant human insulin demonstrated, in six streptozotocin-induced type 1 diabetic mice, a prolonged self-regulated profile of insulin release as a function of glucose concentration for encapsulated microgel [20]. Another in vivo microdevice tested in rats, GRI consisted of an albumin-based bioinorganic membrane that utilizes GOx/CAT and manganese dioxide nanoparticles: during in vitro testing, the microdevices showed glucose-responsive insulin release over multiple cycles at clinically relevant glucose concentrations. In vivo, the microdevices were able to counter hyperglycaemia in diabetic rats over a one-week period [21].
An alternative strategy of achieving a pH responsive release of insulin is to encapsulate insulin inside an acid-disintegrable formulation, such as hydrogels, liposomes, and polymeric vesicles [4][22]. A positively charged acid-disintegrable microgel loaded with insulin by electrostatic interactions and covalently immobilized with GOx and catalase by inverse emulsion polymerization was formulated, aiming for a glucose-regulated insulin release by utilizing GOx/catalase cascade enzymatic reactions to trigger local pH decrease. A local pH decrease within the microgels leads to the diminishment of the net surface negative charges of encapsulated insulin, with a consequent insulin release at high glucose levels [22]. Using chemically modified dextran as an acid-degradable and biocompatible matrix material, a nano-network for glucose-regulated insulin delivery was prepared. The nanoparticles used to form this nano-network comprised four components: an acid-degradable polymeric matrix, polyelectrolyte-based surface coatings, encapsulated glucose-specific enzymes (GOx and catalase), and recombinant insulin. Dextran was selected due to its biocompatibility and biodegradability. The degradation of the nano-network with insulin release was glucose-mediated (facilitated in hyperglycaemia and inhibited in normal glucose levels) in both in vitro and in vivo studies. The blood glucose levels of diabetic mice treated with one injection of nano-network were stably maintained in the normoglycaemia (<200 mg/dL) range for up to 10 days without peaks of hyperglycaemic or hypoglycaemic states [23].
Thus, compared with ConA and PBA systems, glucose-oxidase coupled systems have shown the most promise in vivo in mice; other studies have shown glucose-sensitive insulin release in animal models, with a duration of efficacy ranging from 24 h to 14 days [24][25][26][27].
5. Glucose-Responsive Microneedle Patches for Diabetes Treatment
Several glucose-responsive microneedle (MN)-array patches have been developed to treat diabetes. Previously, open-loop MN-arrays [28][29] were described, but only a closed-loop MN-array could secrete a certain amount of insulin proportional to glucose concentration [30]. In practice, a GRI-MN-array could represent the ideal approach for smart insulin delivery.
Yu et al. [31] reported a GRI delivery device using a painless MN-array patch (“smart insulin patch”) containing glucose-responsive vesicles which were loaded with insulin and GOx enzymes. The vesicles were self-assembled from hypoxia-sensitive hyaluronic acid conjugated with 2-nitroimidazole, a hydrophobic component that can be converted to hydrophilic 2-aminoimidazoles through bioreduction under hypoxic conditions. The local hypoxic microenvironment caused by the enzymatic oxidation of glucose in the hyperglycaemic state promoted the reduction of hypoxia-sensitive hyaluronic acid, which rapidly triggered the dissociation of vesicles and subsequent release of insulin. Vesicles were loaded into a MN-array patch and disassembled in case of interstitial hyperglycaemia with insulin delivery. The smart insulin patch effectively regulated the blood glucose in (STZ)-induced diabetic mice [31].
More recently, several types of MN-array patches have been described. For example, a dissolvable MN patch was fabricated using pullulan, a water-soluble polysaccharide with excellent film-forming ability. After application into human abdominal skin in vitro, the microneedles dissolved within 2 h, releasing up to 87% of insulin. When stored at 4 °C, 20 °C, and 40 °C for 4 weeks, insulin was able to retain its secondary structure, and these MNs were non-cytotoxic toward human keratinocytes [32]. Another dissolving and glucose-responsive insulin-releasing MN patch system was formulated with dissolving and biodegradable gelatine and starch materials, which encapsulated glucose-responsive insulin-releasing gold nanocluster nanocarriers. The gold nanocluster nanocarrier drugs enabled the MN patches to obtain glucose-responsive insulin-releasing behaviour. In in vivo studies, one transdermal application of this patch regulated glycaemia in type 1 diabetic mice in the euglycemic range for 24–48 h [33]. Also, PBA was employed for preparing a MN patch: a purified polymer-bearing pendant PBA motif was combined with a multivalent diol crosslinker to prepare dynamic-covalent hydrogel networks that could be loaded into an MN-array. Insulin release from these materials was accelerated in the presence of glucose, and this activity was confirmed in a diabetic rat model [34] Moreover, short-term blood glucose control in a diabetic rat model following the application of the device to the skin confirms insulin activity and bioavailability. Accordingly, dynamic-covalent crosslinking facilitates a route for fabricating microneedle arrays circumventing the toxicity concerns of in situ polymerization, offering a convenient device form factor for therapeutic insulin delivery [34].
So far, one of the major concerns for the clinical application of MN-array patches is the complexity of the manufacturing process. Very recently, three-dimensional printing technology was employed for the fast fabrication of MN patches. This technique can precisely print microneedles in a few seconds. Thus, 3D-constructed MN patches can efficiently deliver insulin into diabetic mice’s skin by injection, resulting in the effective control of blood glucose levels [35].
In summary, the most studied approaches for developing glucose-responsive insulin delivery have been those based on glucose oxidase, owing to its high specificity for glucose, its current usage in glucose sensors, and the wide array of pH-responsive materials. However, the enzymatic conversion of glucose remains unreliable and slow, and the release of insulin from these nanoparticles is inversely related to glucose concentrations. Glucose binding proteins provide high specificity and binding to glucose; however, limited progress has been made toward eliminating foreign body immune responses. Although small-molecule binders lack glucose specificity, new approaches such as multiplexing PBAs are being explored to address these limitations.
6. Clinical Use of GRI Systems
At present, there is no clinical application for smart insulins. In 2010, Merck & Co., Inc. acquired a smart insulin called MK-2640. MK-2640 is an insulin analogue able to bind the lectin receptor mannose receptor C-type 1 (MRC1). The competitive binding between MK-2640 and glucose to MRC1 was exploited to tune the blood clearance rate of MK-2640 [36][37]. Despite promising results in studies with minipigs and dogs [38], results in humans have failed, leading to the discontinuation of the trial. Compared to pre-clinical studies, MK-2640 did not display a glucose-dependent change in MK-2640 systemic clearance in T1D subjects between euglycemic and hyperglycaemic conditions [39].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241713139
References
- Ravaine, V.; Ancla, C.; Catargi, B. Chemically controlled closed-loop insulin delivery. J. Control Release 2008, 132, 2–11.
- Hoeg-Jensen, T.; Ridderberg, S.; Havelund, S.; Schäffer, L.; Balschmidt, P.; Jonassen, I.; Vedsø, P.; Olesen, P.H.; Markussen, J. Insulins with built-in glucose sensors for glucose responsive insulin release. J. Pept. Sci. 2005, 11, 339–346.
- Jarosinski, M.A.; Dhayalan, B.; Rege, N.; Chatterjee, D.; Weiss, M.A. ‘Smart’ insulin-delivery technologies and intrinsic glucose-responsive insulin analogues. Diabetologia 2021, 64, 1016–1029.
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv. Mater. 2023, 32, e1902004.
- Bawa, P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001.
- Brownlee, M.; Cerami, A. A glucose-controlled insulin-delivery system: Semisynthetic insulin bound to lectin. Science 1979, 206, 1190–1191.
- Brownlee, M.; Cerami, A. Glycosylated insulin complexed to Concanavalin A. Biochemical basis for a closed-loop insulin delivery system. Diabetes 1983, 32, 499–504.
- Nakamae, K.; Miyata, T.; Jikihara, A.; Hoffman, A.S. Formation of poly(glucosyloxyethyl methacrylate)-concanavalin A complex and its glucose-sensitivity. J. Biomater. Sci. Polym. Ed. 1994, 6, 79–90.
- Miyata, T.; Jikihara, A.; Nakamae, K.; Hoffman, A.S. Preparation of poly (2-glucosyloxyethyl methacrylate)-concanavalin A complex hydrogel and its glucose-sensitivity. Macromol. Chem. Physic. 1996, 197, 1135–1146.
- Obaidat, A.A.; Park, K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials 1997, 18, 801–806.
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57.
- Ballerstadt, R.; Evans, C.; McNichols, R.; Gowda, A. Concanavalin A for in vivo glucose sensing: A biotoxicity review. Biosens. Bioelectron. 2006, 22, 275–284.
- Wu, X.; Li, Z.; Chen, X.-X.; Fossey, J.S.; James, T.D.; Jiang, Y.-B. Selective sensing of saccharides using simple boronic acids and their aggregates. Chem. Soc. Rev. 2013, 42, 8032–8048.
- Jonassen, I.; Havelund, S.; Hoeg-Jensen, T.; Steensgaard, D.B.; Wahlund, P.O.; Ribel, U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm. Res. 2012, 29, 2104–2114.
- Chou, D.H.; Webber, M.J.; Tang, B.C.; Lin, A.B.; Thapa, L.S.; Deng, D.; Truong, J.V.; Cortinas, A.B.; Langer, R.; Anderson, D.G. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl. Acad. Sci. USA 2015, 112, 2401–2406.
- Matsumoto, A.; Tanaka, M.; Matsumoto, H.; Ochi, K.; Moro-Oka, Y.; Kuwata, H.; Yamada, H.; Shirakawa, I.; Miyazawa, T.; Ishii, H.; et al. Synthetic “smart gel” provides glucose-responsive insulin delivery in diabetic mice. Sci. Adv. 2017, 3, eaaq0723.
- Ferri, S.; Kojima, K.; Sode, K. Review of glucose oxidases and glucose dehydrogenases: A bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076.
- Ishihara, K.; Kobayashi, M.; Ishimaru, N.; Shinohara, I. Glucose Induced Permeation Control of Insulin through a Complex Membrane Consisting of Immobilized Glucose Oxidase and a Poly(amine). Polym. J. 1984, 16, 625–631.
- Peppas, N.; Hilt, J.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360.
- Gu, Z.; Dang, T.T.; Ma, M.; Tang, B.C.; Cheng, H.; Jiang, S.; Dong, Y.; Zhang, Y.; Anderson, D.G. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano 2013, 7, 6758–6766.
- Chu, M.K.; Chen, J.; Gordijo, C.R.; Chiang, S.; Ivovic, A.; Koulajian, K.; Giacca, A.; Wu, X.Y.; Sun, Y. In vitro and in vivo testing of glucose-responsive insulin-delivery microdevices in diabetic rats. Lab Chip 2012, 12, 2533–2539.
- Wu, Y.; Hu, H.; Hu, J.; Liu, S. Glucose-regulated insulin release from acid-disintegrable microgels covalently immobilized with glucose oxidase and catalase. Macromol. Rapid Commun. 2012, 33, 1852–1860.
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 2013, 7, 4194–4201.
- Traitel, T.; Cohen, Y.; Kost, J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials 2000, 21, 1679–1687.
- Chen, X.; Wu, W.; Guo, Z.; Xin, J.; Li, J. Controlled insulin release from glucose-sensitive self-assembled multilayer films based on 21-arm star polymer. Biomaterials 2011, 32, 1759–1766.
- Kashyap, N.; Viswanad, B.; Sharma, G.; Bhardwaj, V.; Ramarao, P.; Ravi Kumar, M.N. Design and evaluation of biodegradable, biosensitive in situ gelling system for pulsatile delivery of insulin. Biomaterials 2007, 28, 2051–2060.
- Tai, W.; Mo, R.; Di, J.; Subramanian, V.; Gu, X.; Buse, J.B.; Gu, Z. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules 2014, 15, 3495–3502.
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915.
- Ling, M.H.; Chen, M.C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961.
- Chen, G.; Yu, J.; Gu, Z. Glucose-Responsive Microneedle Patches for Diabetes Treatment. J. Diabetes Sci. Technol. 2019, 13, 41–48.
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265.
- Fonseca, D.F.S.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Duarte-Araújo, M.; Morato, M.; Vilela, C.; et al. Pullulan microneedle patches for the efficient transdermal administration of insulin envisioning diabetes treatment. Carbohydr. Polym. 2020, 241, 116314.
- Zhang, Y.; Wu, M.; Tan, D.; Liu, Q.; Xia, R.; Chen, M.; Liu, Y.; Xue, L.; Lei, Y. A dissolving and glucose-responsive insulin releasing microneedle patch for type 1 diabetes therapy. J. Mater. Chem. B 2021, 9, 648–657.
- Ye, Z.; Xiang, Y.; Monroe, T.; Yu, S.; Dong, P.; Xian, S.; Webber, M.J. Polymeric Microneedle Arrays with Glucose-Sensing Dynamic-Covalent Bonding for Insulin Delivery. Biomacromolecules 2022, 23, 4401–4411.
- Li, R.; Liu, X.; Yuan, X.; Wu, S.; Li, L.; Jiang, X.; Li, B.; Jiang, X.; Gou, M. Fast Customization of Hollow Microneedle Patches for Insulin Delivery. Int. J. Bioprint. 2022, 8, 553.
- Yang, R.; Wu, M.; Lin, S.; Nargund, R.P.; Li, X.; Kelly, T.; Yan, L.; Dai, G.; Qian, Y.; Dallas-Yang, Q.; et al. A glucose-responsive insulin therapy protects animals against hypoglycemia. JCI Insight 2018, 3, e97476.
- Zeng, Y.; Wang, J.; Gu, Z.; Gu, Z. Engineering glucose-responsive insulin. Med. Drug Discov. 2019, 3, 100010.
- Kaarsholm, N.C.; Lin, S.; Yan, L.; Kelly, T.; van Heek, M.; Mu, J.; Wu, M.; Dai, G.; Cui, Y.; Zhu, Y.; et al. Engineering Glucose Responsiveness into Insulin. Diabetes 2018, 67, 299–308.
- Krug, A.W.; Visser, S.A.G.; Tsai, K.; Kandala, B.; Fancourt, C.; Thornton, B.; Morrow, L.; Kaarsholm, N.C.; Bernstein, H.S.; Stoch, S.A.; et al. Clinical Evaluation of MK-2640: An Insulin Analog with Glucose-Responsive Properties. Clin. Pharmacol. Ther. 2018, 105, 417–425.
This entry is offline, you can click here to edit this entry!
