Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Global climate change and population growth are persistently posing threats to natural resources (e.g., freshwater) and agricultural production. Crassulacean acid metabolism (CAM) evolved from C3 photosynthesis as an adaptive form of photosynthesis in hot and arid regions. It features the nocturnal opening of stomata for CO2 assimilation, diurnal closure of stomata for water conservation, and high water-use efficiency.
- Crassulacean acid metabolism
- C3 to CAM transition
- facultative CAM
1. Introduction
Drastic climate change over the past decades can be reflected by the alternation in atmospheric CO2 levels, tropospheric ozone concentrations, and other environmental indicators [1]. Climate change is not only affecting ecosystems, but also agriculture, food production, land, and water resources [2]. Arid or semi-arid land accounts for around 41% of the total surface on Earth, and it is expanding [3]. In 2035, global desertification is projected to be 65% of the total land surface in the subtropical regions [4]. With the rapid growth of the human population, the demand for food is increasing, and it is anticipated to surge by 70%. The current rate of global crop productivity only increases by ~2% per year, which cannot meet the demand for food [5]. To worsen the situation, the global decrease in freshwater from 1980 to 2015 has caused a 20.6% and 39.3% yield reduction in wheat and maize, respectively [6].
Photosynthesis, a pivotal biological process essential to all life, provides food and most of our energy resources [7]. There are three major modes of photosynthesis in vascular plants to assimilate atmospheric CO2: C3, C4, and Crassulacean acid metabolism (CAM) [8,9] (Figure 1). CAM photosynthesis has evolved independently multiple times from C3 as a photosynthetic adaptation to cope with the decreasing atmospheric CO2 levels ~20 million years ago [10]. CAM plants are commonly found in harsh environments such as arid and semi-arid regions [11]. Other than those water-limited regions, CAM plants also inhabit the aquatic environment. With the release of the genome and transcriptome of an underwater CAM plant Isoetes taiwanensis [12], differences in the recruitment of phosphoenolpyruvate (PEP) carboxylase (PEPC) and core CAM pathway gene expression between aquatic and terrestrial plants demonstrate a different route of CAM evolution.
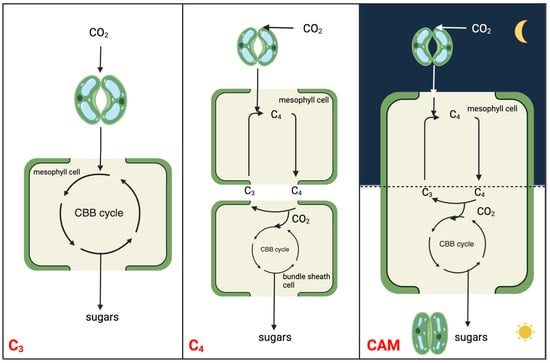
Figure 1. Simplified schematic to illustrate the molecular relationships and distinctions among C3, C4, and CAM photosynthesis mechanisms. The CBB cycle is the abbreviation of Calvin-Benson-Bassham cycle, which is also known as Calvin cycle.
CAM is a carbon concentrating mechanism, with the capability of assimilating CO2 initially at night using PEPC in the cytosol, leading to the formation of a four-carbon malate, which is then stored in the vacuole [13,14]. The three-carbon acceptor in this reaction is PEP, which is replenished by the glycolytic breakdown of carbohydrate storage in the form of starch or other sugars. Unlike spatial decoupling of carboxylation and decarboxylation in C4 photosynthesis, CAM photosynthesis separates these two processes in a temporal manner to shield ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCO) from the oxygenase activity, minimizing photorespiration (Figure 1). CAM plants conduct gas exchange predominantly at night when the air temperature is low, thereby having a lower water loss by an order of magnitude than it would be during the day [15]. As such, CAM plants have water-use efficiency (WUE) several-fold higher than those of C3 and C4 plants under comparable conditions [16]. High WUE, together with enhanced heat and drought tolerance, drives the basic and applied research on CAM toward crop CAM engineering/bio-design. The typical diel cycle of CAM entails four phases: (I) nocturnal atmospheric CO2 fixation by PEPC and malic acid storage in the vacuole; (II) RuBisCO activation just after dawn when, for a brief period, CO2 is fixed by both PEPC and RuBisCO; (III) stored malate decarboxylated to CO2, which is fixed by RuBisCO; (IV) the end of the light period when stomata reopen driven by the depletion of malate pool [13,17].
CAM plants normally exhibit the following features: the diurnal fluctuation of malic acids (accumulation during the night period and dissipation during the day); reciprocal diurnal fluctuation of storage carbohydrates such as starch, polyglucans, or soluble hexoses; a high level of PEPC and an active decarboxylase; large storage vacuoles that are in the same cells with chloroplasts; water-limitation related traits, such as dense trichomes, leaf succulence, and waxy cuticles [1]; and nocturnal net CO2 uptake, which exhibits an inverse pattern of stomatal movement [18]. In the course of evolution, an intermediate CAM mechanism called facultative CAM arose. Plants conducting facultative CAM demonstrate the optional use of CAM under stress conditions, while remaining the use of C3 or C4 photosynthesis under normal conditions [19,20,21,22,23].
Given the characteristics of facultative CAM photosynthesis and climate change urgency, unraveling the molecular mechanisms underlying the C3 to CAM transition has attracted growing interest. However, there is a lack of review on facultative CAM and especially C3 to CAM transition. Herein we summarize recent advances in facultative C3 to CAM transition, discuss current problems and challenges, and highlight future research directions (Figure 2).
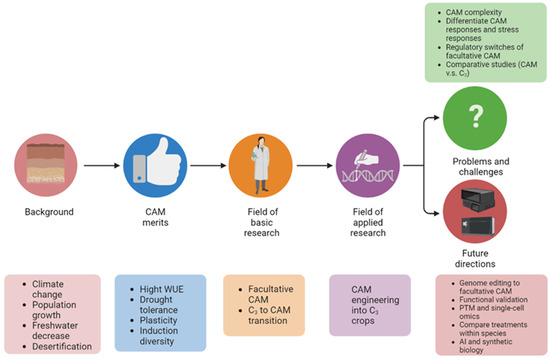
Figure 2. Graphical summary of the significance of studying C3 to CAM transition of facultative CAM plants. The abbreviations used: C3, C3 photosynthesis; CAM, Crassulacean acid metabolism; WUE, water-use efficiency; and PTM, post-translational modification.
2. The Plasticity of CAM Is Best Represented in Facultative CAM
A remarkable hallmark of CAM plants is their considerable plasticity in expressing the four phases of CAM, while keeping the C3 cycle fully functional [24]. Environmental factors such as light intensity, relative humidity, water availability [25], and developmental stages [16] affect the degree and duration of CAM expression [9]. CAM is highly plastic and can operate in different modes: (1) obligate/constitutive CAM or strong CAM, with high nocturnal acid accumulation (ΔH+) and CO2 fixation; (2) CAM-cycling, with daytime CO2 fixation like C3 and nocturnal fixation of CO2 from respiration; (3) CAM idling, with stomata closed all the time and CAM fixation of CO2 only from respiration; (4) facultative/inducible CAM, with C3 mode of CO2 fixation and zero ΔH+ in the non-stressed state, and small nocturnal CO2 fixation and ΔH+ during C3 to CAM transition in the stressed state [26]; (5) weak CAM, with similar CO2 uptake pattern as strong CAM but less nocturnal acid accumulation.
Among the above five different modes of CAM, a preeminent model for elucidating the molecular underpinnings of CAM is facultative CAM [27,28]. In facultative CAM species, CAM may be induced by a variety of stimuli such as drought [29,30], salinity [19,31], high photosynthetic photon flux [6,32], abscisic acid (ABA) [33], photoperiod [34] and hydrogen peroxide [35]. Clearly, CAM plasticity is best represented by facultative CAM plants, which employ the C3 photosynthesis under non-stress conditions to maximize growth, but are able to undergo a gradual C3 to CAM transition to reduce water loss and maintain photosynthetic integrity under water-limited conditions. It ultimately translates into high WUE, survival, and reproductive success [36]. Facultative CAM plants have been identified in a wide range of plant families, such as Bromeliaceae, Cactaceae, Aizoaceae, Montiaceae, Lamiaceae, Vitaceae, and Didiereaceae [37], indicating multiple independent evolutionary events (Figure 3). Whether these independent events generated similar or different genetic and epigenetic changes that enable facultative CAM deserves immediate investigation, e.g., by identifying and utilizing the evolutionary pairs of C3 and CAM species.
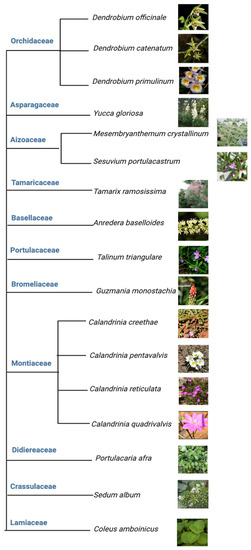
Figure 3. Tree view of all the facultative CAM plants investigated from 2017 to 2023.
3. Studies on C3 to CAM Transition Revealed Important Molecular Players
Over the past decades, different model species were used for diverse aspects of research pertinent to CAM. Here are six main areas of CAM research: (1) CAM ecophysiology to study and discover new CAM species in different ecological environments (e.g., [27,54]); (2) CAM origin and evolution (e.g., [55,56]); (3) genomic features and molecular mechanisms regulating CAM (e.g., [57,58]); (4) C3 to CAM transition (e.g., [19,20]); (5) CAM metabolic modeling (e.g., [59,60]); and (6) engineering CAM into C3 plants (e.g., [5,61]). All these areas of basic research aim for the ultimate goal of exploiting the potential of CAM in crop improvement under climate change [56]. Based on recent publications, Ananas comosus (Pineapple), Kalanchoë fedtschenkoi, and Mesembryanthemum crystallinum (Common ice plant, Table 1) are the three most extensively studied CAM models. Their genomes have been fully sequenced [58,62,63]. For studying the C3 to CAM transition, M. crystallinum has been the classic model, and Talinum triangulare is an emerging model [17]. T. triangulare is an herbaceous weed that shifts from C3 to CAM photosynthesis on day 11 of drought treatment. The large evenly green leaves, rapid growth, relatively short life cycle, self-cross, and full reversibility of CAM make it a model system to study facultative CAM [28].
Table 1. Research progress on CAM in M. crystallinum from 2017 to 2022.
| Research Focus | Key Findings | Reference |
|---|---|---|
| Identified C3-CAM transition period and temporal physiological changes |
|
[20] |
| Transcriptomics of guard cells during the C3-CAM transition |
|
[19] |
|
||
| Nocturnal carboxylation is coordinated with starch degradation by the products of these pathways, such as carbohydrates |
|
[64] |
| Functional CAM withdrawal in the de-salted plants |
|
[65] |
| Comparative proteomic changes in guard cells and mesophyll cells during the C3-CAM transition |
|
[66] |
| Phytohormones in the stomatal behavior during the C3-CAM transition |
|
[67] |
| Genome sequencing, transcriptomics, and comparative genomics of leaves |
|
[62] |
The early research of C3 to CAM transition mainly focused on several key CAM enzymes, such as PEPC [68], PEPC protein kinase (PPCK) and malic enzymes, as well as metabolite transport in the C3 and CAM state [69,70]. Early studies suggest that ABA signaling, Ca2+ signaling, and protein phosphorylation/de-phosphorylation may play important roles in the C3 to CAM transition, whereas no key players such as kinases/phosphatases were identified. Sequence analyses were also performed on PPC1 and PPC2, which are CAM-specific genes that encode PEPC [71,72]. With the emergence of microarray technologies, large-scale mRNA profiling was carried out [73]. Instead of studying the transition, Cushman group compared the gene expression of ice plants between the non-stressed C3 group and the induced-CAM group after 14 days of salt treatment. Gene expression of eight transporters was analyzed to study the inter-organellar metabolite transport between C3 and the CAM group of ice plants [74]. However, there is a lack of understanding of the temporal metabolic and molecular control of the C3 to CAM transition and a systems-level understanding was needed to reveal the regulatory changes underlying the transition.
With the advances in high-throughput omics technologies and computational biology, systems biology has become a prevalent approach for discovery (hypothesis generation) and functional studies (hypothesis testing). Beyond traditional physiological and biochemical methods, multi-omics (genomics, transcriptomics, proteomics and metabolomics) has generated a systems-level understanding of temporal molecular and metabolic controls underpinning CAM [75]. In T. triangulare leaves, targeted metabolite profiling and RNA sequencing were performed to reveal the rewiring of carbohydrate metabolism and candidate transcription factors (TFs) in the drought-induced CAM transition process [76]. Three years later, the same group identified seven candidate regulators of ABA-induced CAM including HEAT SHOCK TF A2, NUCLEAR FACTOR Y, SUBUNITS A9, and JmjC DOMAIN-CONTAINING PROTEIN 27 [77]. In addition to the traditional drought and salt induction of CAM, hydrogen peroxide was shown to be able to induce CAM in M. crystallinum [35]. In the leaves of another facultative bromeliad Guzmania monostachia, increases in the expression of CAM-related genes (PEPC1, PPCK, NAD-malate dehydrogenase, aluminum-activated malate transporter 9 (ALMT9), PEP carboxykinase (PEPCK)) and UREASE transcripts were shown under drought. And the role of integrating N and C metabolism of urea was suggested [78]. The CAM gene expression, antioxidant activities, and chlorophyll fluorescence were compared between a C3-CAM facultative species (Sedum album) and a C4-CAM facultative species (Portulaca oleracea) [79]. The level of nitric oxide (NO) was found to be correlated with the CAM expression during CAM induction only in S. album but not P. oleracea. This suggests the different roles of NO in C3 and C4 species during CAM induction. All the aforementioned studies did not identify a critical transition period, which is key to capturing the molecular switches for CAM. Three years ago, the transition period of M. crystallinum was first defined during salt-induced C3 to CAM shift, and further validated in independent studies through RNA-seq and physiological analyses [19,20,67]. Interestingly, three phytohormones, jasmonic acid (JA), cytokinin, and ABA were reported to play important roles in the inversed pattern of stomata opening/closing during the transition of M. crystallinum [67]. With the release of the ice plant genome [62], more studies can explore the genes and metabolites pertinent to the C3 to CAM transition.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241713072
This entry is offline, you can click here to edit this entry!
