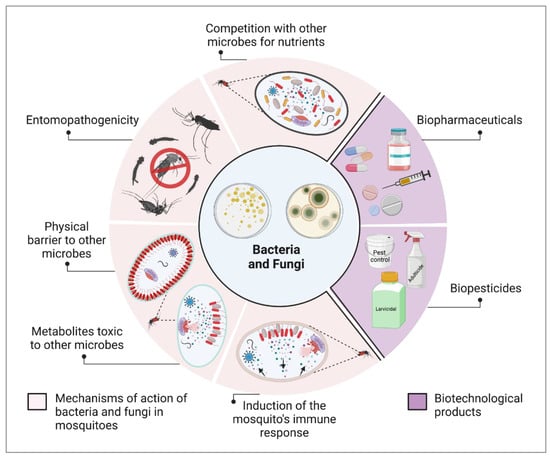
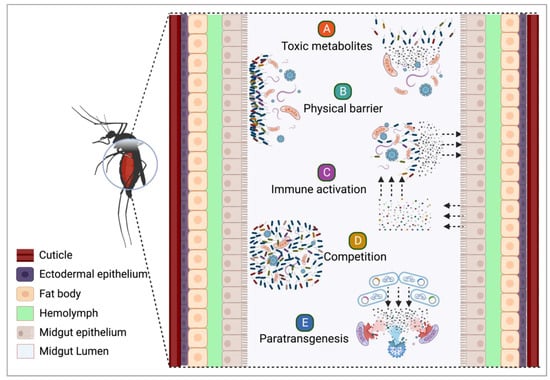
Mosquitoes transmit pathogens that cause human diseases such as malaria, dengue fever, chikungunya, yellow fever, Zika fever, and filariasis. Biotechnological approaches using microorganisms have a significant potential to control mosquito populations and reduce their vector competence, making them alternatives to synthetic insecticides. Ongoing research has identified many microorganisms that can be used effectively to control mosquito populations and disease transmission. However, the successful implementation of these newly proposed approaches requires a thorough understanding of the multipronged microorganism–mosquito–pathogen–environment interactions. Although much has been achieved in discovering new entomopathogenic microorganisms, antipathogen compounds, and their mechanisms of action, only a few have been turned into viable products for mosquito control. There is a discrepancy between the number of microorganisms with the potential for the development of new insecticides and/or antipathogen products and the actual available products, highlighting the need for investments in the intersection of basic research and biotechnology.
- biotechnology
- microorganisms
- bacteria
- fungi
- vector control
- mosquitoes
1. Introduction
2. Symbiotic Bacteria and Their Potential against Infectious Agents
3. Wolbachia-Based Strategy for Controlling Mosquito-Borne Viruses: Mechanisms, Efficacy, and Implications
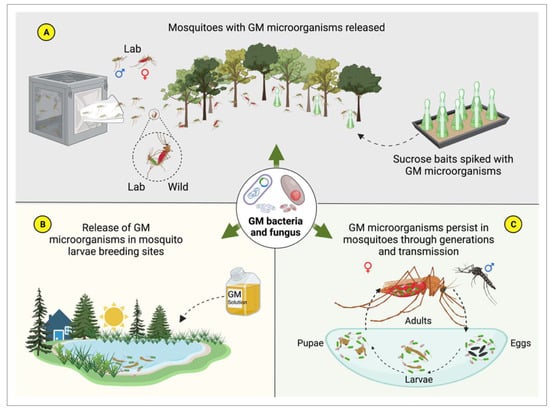
4. Symbiotic Microorganisms and Paratransgenesis
This entry is adapted from the peer-reviewed paper 10.3390/insects14090718
References
- Onen, O.; Aboh, A.A.; Mfam, A.N.; Akor, M.O.; Nweke, C.N.; Osuagwu, A.N. Microbial Diversity: Values and Roles in Ecosystems. Asian J. Biol. 2020, 9, 10–22.
- Rousk, J.; Bengtson, P. Microbial Regulation of Global Biogeochemical Cycles. Front. Microbiol. 2014, 5, 103.
- Rodríguez-Frías, F.; Quer, J.; Tabernero, D.; Cortese, M.F.; Garcia-Garcia, S.; Rando-Segura, A.; Pumarola, T. Microorganisms as Shapers of Human Civilization, from Pandemics to Even Our Genomes: Villains or Friends? A Historical Approach. Microorganisms 2021, 9, 2518.
- Raaijmakers, J.M.; Vlami, M.; De Souza, J.T. Antibiotic Production by Bacterial Biocontrol Agents. Antonie Leeuwenhoek 2002, 81, 537–547.
- Uchida, R.; Imasato, R.; Tomoda, H.; Ōmura, S. Yaequinolones, New Insecticidal Antibiotics Produced by Penicillium sp. FKI-2140. J. Antibiot. 2006, 59, 652–658.
- Bintsis, T. Lactic Acid Bacteria: Their Applications in Foods. J. Bacteriol. Mycol. 2018, 6, 89–94.
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for Future Foods. J. Future Foods 2021, 1, 25–37.
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial Interactions in Alcoholic Beverages. Int. Microbiol. 2022, 25, 1–15.
- Sevak, P.I.; Pushkar, B.K.; Kapadne, P.N. Lead Pollution and Bacterial Bioremediation: A Review. Environ. Chem. Lett. 2021, 19, 4463–4488.
- Cowan, A.R.; Costanzo, C.M.; Benham, R.; Loveridge, E.J.; Moody, S.C. Fungal Bioremediation of Polyethylene: Challenges and Perspectives. J. Appl. Microbiol. 2022, 132, 78–89.
- Jiménez-Gómez, A.; García-Estévez, I.; Escribano-Bailón, M.T.; García-Fraile, P.; Rivas, R. Bacterial Fertilizers Based on Rhizobium laguerreae and Bacillus halotolerans Enhance Cichorium endivia L. Phenolic Compound and Mineral Contents and Plant Development. Foods 2021, 10, 424.
- Dunham, B. Microbial Pesticides: A Key Role in the Multinational Portfolio. New Ag Int. 2015, 32–36.
- Ruiu, L. Microbial Biopesticides in Agroecosystems. Agronomy 2018, 8, 235.
- Thongsripong, P.; Chandler, J.A.; Green, A.B.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Mosquito Vector-associated Microbiota: Metabarcoding Bacteria and Eukaryotic Symbionts across Habitat Types in Thailand Endemic for Dengue and Other Arthropod-borne Diseases. Ecol. Evol. 2018, 8, 1352–1368.
- da Silva Gonçalves, D.; Iturbe-Ormaetxe, I.; Martins-da-Silva, A.; Telleria, E.L.; Rocha, M.N.; Traub-Csekö, Y.M.; O’Neill, S.L.; Sant’Anna, M.R.V.; Moreira, L.A. Wolbachia Introduction into Lutzomyia longipalpis (Diptera: Psychodidae) Cell Lines and Its Effects on Immune-Related Gene Expression and Interaction with Leishmania infantum. Parasites Vectors 2019, 12, 33.
- Caragata, E.P.; Short, S.M. Vector Microbiota and Immunity: Modulating Arthropod Susceptibility to Vertebrate Pathogens. Curr. Opin. Insect Sci. 2022, 50, 100875.
- Dennison, N.J.; Jupatanakul, N.; Dimopoulos, G. The Mosquito Microbiota Influences Vector Competence for Human Pathogens. Curr. Opin. Insect Sci. 2014, 3, 6–13.
- Carlson, J.S.; Short, S.M.; Angleró-Rodríguez, Y.I.; Dimopoulos, G. Larval Exposure to Bacteria Modulates Arbovirus Infection and Immune Gene Expression in Adult Aedes aegypti. Dev. Comp. Immunol. 2020, 104, 103540.
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito Microbiota and Implications for Disease Control. Trends Parasitol. 2020, 36, 98–111.
- Gabrieli, P.; Caccia, S.; Varotto-Boccazzi, I.; Arnoldi, I.; Barbieri, G.; Comandatore, F.; Epis, S. Mosquito Trilogy: Microbiota, Immunity and Pathogens, and Their Implications for the Control of Disease Transmission. Front. Microbiol. 2021, 12, 630438.
- Cansado-Utrilla, C.; Zhao, S.Y.; McCall, P.J.; Coon, K.L.; Hughes, G.L. The Microbiome and Mosquito Vectorial Capacity: Rich Potential for Discovery and Translation. Microbiome 2021, 9, 111.
- Wang, J.; Gao, L.; Aksoy, S. Microbiota in Disease-Transmitting Vectors. Nat. Rev. Microbiol. 2023, 21, 604–618.
- Douglas, A.E. Lessons from Studying Insect Symbioses. Cell Host Microbe 2011, 10, 359–367.
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and Function of Bacterial Microbiota in the Mosquito Holobiont. Parasites Vectors 2013, 6, 146.
- Kumar, A.; Srivastava, P.; Sirisena, P.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito Innate Immunity. Insects 2018, 9, 95.
- Ferreira, Q.R.; Lemos, F.F.B.; Moura, M.N.; de Nascimento, J.O.S.; Novaes, A.F.; Barcelos, I.S.; Fernandes, L.A.; de Amaral, L.S.B.; Barreto, F.K.; de Melo, F.F. Role of the Microbiome in Aedes spp. Vector Competence: What Do We Know? Viruses 2023, 15, 779.
- Saab, S.A.; Dohna, H.Z.; Nilsson, L.K.; Onorati, P.; Nakhleh, J.; Terenius, O.; Osta, M.A. The Environment and Species Affect Gut Bacteria Composition in Laboratory Co-Cultured Anopheles gambiae and Aedes albopictus Mosquitoes. Sci. Rep. 2020, 10, 3352.
- Mosquera, K.D.; Nilsson, L.K.J.; de Oliveira, M.R.; Rocha, E.M.; Marinotti, O.; Håkansson, S.; Tadei, W.P.; de Souza, A.Q.L.; Terenius, O. Comparative Assessment of the Bacterial Communities Associated with Anopheles darlingi Immature Stages and Their Breeding Sites in the Brazilian Amazon. Parasites Vectors 2023, 16, 156.
- Dos Santos, N.A.C.; de Carvalho, V.R.; Souza Neto, J.; Alonso, D.P.; Ribolla, P.E.M.; Medeiros, J.F.; da Araujo, M.S. Bacterial Microbiota from Lab-Reared and Field-Captured Anopheles darlingi Midgut and Salivary Gland. Microorganisms 2023, 11, 1145.
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the Mosquito Midgut Microbiota in the Defense against Malaria Parasites. PLoS Pathog. 2009, 5, e1000423.
- Cirimotich, C.M.; Dong, Y.; Garver, L.S.; Sim, S.; Dimopoulos, G. Mosquito Immune Defenses against Plasmodium Infection. Dev. Comp. Immunol. 2010, 34, 387–395.
- Wang, Y.; Gilbreath III, T.M.; Kukutla, P.; Yan, G.; Xu, J. Dynamic Gut Microbiome across Life History of the Malaria Mosquito Anopheles gambiae in Kenya. PLoS ONE 2011, 6, e24767.
- Gendrin, M.; Christophides, G.K. The Anopheles Mosquito Microbiota and Their Impact on Pathogen Transmission. In Anopheles Mosquitoes-New Insights into Malaria Vectors; IntechOpen: Rijeka, Croatia, 2013; ISBN 953-51-1188-4.
- Ricci, I.; Valzano, M.; Ulissi, U.; Epis, S.; Cappelli, A.; Favia, G. Symbiotic Control of Mosquito Borne Disease. Pathog. Glob. Health 2012, 106, 380–385.
- Eappen, A.G.; Smith, R.C.; Jacobs-Lorena, M. Enterobacter-Activated Mosquito Immune Responses to Plasmodium Involve Activation of SRPN6 in Anopheles stephensi. PLoS ONE 2013, 8, e62937.
- Romoli, O.; Gendrin, M. The Tripartite Interactions between the Mosquito, Its Microbiota and Plasmodium. Parasites Vectors 2018, 11, 200.
- Shi, C.; Beller, L.; Wang, L.; Rosales Rosas, A.; De Coninck, L.; Héry, L.; Mousson, L.; Pagès, N.; Raes, J.; Delang, L. Bidirectional Interactions between Arboviruses and the Bacterial and Viral Microbiota in Aedes aegypti and Culex quinquefasciatus. MBio 2022, 13, e01021-22.
- Pumpuni, C.B.; Beier, M.S.; Nataro, J.P.; Guers, L.D.; Davis, J.R. Plasmodium falciparum: Inhibition of Sporogonic Development in Anopheles stephensi by Gram-Negative Bacteria. Exp. Parasitol. 1993, 77, 195–199.
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural Microbe-Mediated Refractoriness to Plasmodium Infection in Anopheles gambiae. Science 2011, 332, 855–858.
- Dennison, N.J.; Saraiva, R.G.; Cirimotich, C.M.; Mlambo, G.; Mongodin, E.F.; Dimopoulos, G. Functional Genomic Analyses of Enterobacter, Anopheles and Plasmodium Reciprocal Interactions That Impact Vector Competence. Malar. J. 2016, 15, 425.
- Bando, H.; Okado, K.; Guelbeogo, W.M.; Badolo, A.; Aonuma, H.; Nelson, B.; Fukumoto, S.; Xuan, X.; Sagnon, N.; Kanuka, H. Intra-Specific Diversity of Serratia marcescens in Anopheles Mosquito Midgut Defines Plasmodium Transmission Capacity. Sci. Rep. 2013, 3, 1641.
- Tchioffo, M.T.; Boissiere, A.; Churcher, T.S.; Abate, L.; Gimonneau, G.; Nsango, S.E.; Awono-Ambene, P.H.; Christen, R.; Berry, A.; Morlais, I. Modulation of Malaria Infection in Anopheles gambiae Mosquitoes Exposed to Natural Midgut Bacteria. PLoS ONE 2013, 8, e81663.
- Bai, L.; Wang, L.; Vega-Rodríguez, J.; Wang, G.; Wang, S. A Gut Symbiotic Bacterium Serratia marcescens Renders Mosquito Resistance to Plasmodium Infection through Activation of Mosquito Immune Responses. Front. Microbiol. 2019, 10, 1580.
- Gao, H.; Bai, L.; Jiang, Y.; Huang, W.; Wang, L.; Li, S.; Zhu, G.; Wang, D.; Huang, Z.; Li, X. A Natural Symbiotic Bacterium Drives Mosquito Refractoriness to Plasmodium Infection via Secretion of an Antimalarial Lipase. Nat. Microbiol. 2021, 6, 806–817.
- Cappelli, A.; Damiani, C.; Mancini, M.V.; Valzano, M.; Rossi, P.; Serrao, A.; Ricci, I.; Favia, G. Asaia Activates Immune Genes in Mosquito Eliciting an Anti-Plasmodium Response: Implications in Malaria Control. Front. Genet. 2019, 10, 836.
- Ramirez, J.L.; Short, S.M.; Bahia, A.C.; Saraiva, R.G.; Dong, Y.; Kang, S.; Tripathi, A.; Mlambo, G.; Dimopoulos, G. Chromobacterium Csp_P Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and in Vitro Anti-Pathogen Activities. PLoS Pathog. 2014, 10, e1004398.
- Ramirez, J.L.; Souza-Neto, J.; Torres Cosme, R.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal Tripartite Interactions between the Aedes aegypti Midgut Microbiota, Innate Immune System and Dengue Virus Influences Vector Competence. PLoS Neglected Trop. Dis. 2012, 6, e1561.
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278.
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P. The w Mel Wolbachia Strain Blocks Dengue and Invades Caged Aedes aegypti Populations. Nature 2011, 476, 450–453.
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The WMel Strain of Wolbachia Reduces Transmission of Zika Virus by Aedes aegypti. Sci. Rep. 2016, 6, 28792.
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L. Establishment of WMel Wolbachia in Aedes aegypti Mosquitoes and Reduction of Local Dengue Transmission in Cairns and Surrounding Locations in Northern Queensland, Australia. Gates Open Res. 2019, 3, 1547.
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.; Arif, M.A.; Thohir, H.; NurSyamimi, H. Establishment of Wolbachia Strain WAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Curr. Biol. 2019, 29, 4241–4248.e5.
- Fraser, J.E.; O’Donnell, T.B.; Duyvestyn, J.M.; O’Neill, S.L.; Simmons, C.P.; Flores, H.A. Novel Phenotype of Wolbachia Strain w Pip in Aedes aegypti Challenges Assumptions on Mechanisms of Wolbachia-Mediated Dengue Virus Inhibition. PLoS Pathog. 2020, 16, e1008410.
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary Cholesterol Modulates Pathogen Blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459.
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed Cholesterol and Vesicular Trafficking Associated with Dengue Blocking in Wolbachia-Infected Aedes aegypti Cells. Nat. Commun. 2017, 8, 526.
- Kambris, Z.; Cook, P.E.; Phuc, H.K.; Sinkins, S.P. Immune Activation by Life-Shortening Wolbachia and Reduced Filarial Competence in Mosquitoes. Science 2009, 326, 134–136.
- Rancès, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The Relative Importance of Innate Immune Priming in Wolbachia-Mediated Dengue Interference. PLoS Pathog. 2012, 8, e1002548.
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021, 384, 2177–2186.
- Pinto, S.B.; Riback, T.I.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A. Effectiveness of Wolbachia-Infected Mosquito Deployments in Reducing the Incidence of Dengue and Other Aedes-Borne Diseases in Niterói, Brazil: A Quasi-Experimental Study. PLoS Neglected Trop. Dis. 2021, 15, e0009556.
- Dodson, B.L.; Pujhari, S.; Brustolin, M.L.; Metz, H.C.; Rasgon, J.L. Variable Effects of Wolbachia on Alphavirus Infection in Aedes aegypti. bioRxiv 2023.
- Loreto, E.L.S.; Wallau, G.L. Risks of Wolbachia Mosquito Control. Science 2016, 351, 1273.
- Sanaei, E.; Charlat, S.; Engelstädter, J. Wolbachia Host Shifts: Routes, Mechanisms, Constraints and Evolutionary Consequences. Biol. Rev. 2021, 96, 433–453.
- Edenborough, K.M.; Flores, H.A.; Simmons, C.P.; Fraser, J.E. Using Wolbachia to Eliminate Dengue: Will the Virus Fight Back? J. Virol. 2021, 95, e02203-20.
- Thi Hue Kien, D.; Edenborough, K.M.; da Silva Goncalves, D.; Thuy Vi, T.; Casagrande, E.; Thi Le Duyen, H.; Thi Long, V.; Thi Dui, L.; Thi Tuyet Nhu, V.; Thi Giang, N. Genome Evolution of Dengue Virus Serotype 1 under Selection by Wolbachia pipientis in Aedes aegypti Mosquitoes. Virus Evol. 2023, 3, vead016.
- Wilke, A.B.B.; Marrelli, M.T. Paratransgenesis: A Promising New Strategy for Mosquito Vector Control. Parasites Vectors 2015, 8, 342.
- Wang, S.; Jacobs-Lorena, M. Paratransgenesis Applications: Fighting Malaria with Engineered Mosquito Symbiotic Bacteria. In Arthropod Vector: Controller of Disease Transmission, Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–234.
- Ratcliffe, N.A.; Furtado Pacheco, J.P.; Dyson, P.; Castro, H.C.; Gonzalez, M.S.; Azambuja, P.; Mello, C.B. Overview of Paratransgenesis as a Strategy to Control Pathogen Transmission by Insect Vectors. Parasites Vectors 2022, 15, 112.
- Wang, S.; Jacobs-Lorena, M. Transgenesis and Paratransgenesis for the Control of Malaria. In Mosquito Gene Drives and the Malaria Eradication Agenda; Jenny Stanford Publishing, United Square: Singapore, 2023; pp. 21–37.
- Huang, W.; Wang, S.; Jacobs-Lorena, M. Use of Microbiota to Fight Mosquito-Borne Disease. Front. Genet. 2020, 11, 196.
- Yoshida, S.; Ioka, D.; Matsuoka, H.; Endo, H.; Ishii, A. Bacteria Expressing Single-Chain Immunotoxin Inhibit Malaria Parasite Development in Mosquitoes. Mol. Biochem. Parasitol. 2001, 113, 89–96.
- Wang, S.; Ghosh, A.K.; Bongio, N.; Stebbings, K.A.; Lampe, D.J.; Jacobs-Lorena, M. Fighting Malaria with Engineered Symbiotic Bacteria from Vector Mosquitoes. Proc. Natl. Acad. Sci. USA 2012, 109, 12734–12739.
- Wang, S.; Dos-Santos, A.L.; Huang, W.; Liu, K.C.; Oshaghi, M.A.; Wei, G.; Agre, P.; Jacobs-Lorena, M. Driving Mosquito Refractoriness to Plasmodium falciparum with Engineered Symbiotic Bacteria. Science 2017, 357, 1399–1402.
- Villegas, L.M.; Pimenta, P.F.P. Metagenomics, Paratransgenesis and the Anopheles Microbiome: A Portrait of the Geographical Distribution of the Anopheline Microbiota Based on a Meta-Analysis of Reported Taxa. Memórias Inst. Oswaldo Cruz 2014, 109, 672–684.
- Bongio, N.J.; Lampe, D.J. Inhibition of Plasmodium berghei Development in Mosquitoes by Effector Proteins Secreted from Asaia sp. Bacteria Using a Novel Native Secretion Signal. PLoS ONE 2015, 10, e0143541.
- Mancini, M.V.; Spaccapelo, R.; Damiani, C.; Accoti, A.; Tallarita, M.; Petraglia, E.; Rossi, P.; Cappelli, A.; Capone, A.; Peruzzi, G. Paratransgenesis to Control Malaria Vectors: A Semi-Field Pilot Study. Parasites Vectors 2016, 9, 140.
- Raharimalala, F.N.; Boukraa, S.; Bawin, T.; Boyer, S.; Francis, F. Molecular Detection of Six (Endo-) Symbiotic Bacteria in Belgian Mosquitoes: First Step towards the Selection of Appropriate Paratransgenesis Candidates. Parasitol. Res. 2016, 115, 1391–1399.
- Rocha, E.M.; Marinotti, O.; Serrão, D.M.; Correa, L.V.; de Katak, R.M.; de Oliveira, J.C.; Muniz, V.A.; de Oliveira, M.R.; do Nascimento Neto, J.F.; Pessoa, M.C.F. Culturable Bacteria Associated with Anopheles darlingi and Their Paratransgenesis Potential. Malar. J. 2021, 20, 40.
- Fang, W.; Vega-Rodríguez, J.; Ghosh, A.K.; Jacobs-Lorena, M.; Kang, A.; St. Leger, R.J. Development of Transgenic Fungi That Kill Human Malaria Parasites in Mosquitoes. Science 2011, 331, 1074–1077.
- Carballar-Lejarazú, R.; Rodriguez, M.H.; de la Cruz Hernández-Hernández, F.; Ramos-Castaneda, J.; Possani, L.D.; Zurita-Ortega, M.; Reynaud-Garza, E.; Hernández-Rivas, R.; Loukeris, T.; Lycett, G. Recombinant Scorpine: A Multifunctional Antimicrobial Peptide with Activity against Different Pathogens. Cell. Mol. Life Sci. 2008, 65, 3081–3092.
- Ward, T.W.; Jenkins, M.S.; Afanasiev, B.N.; Edwards, M.; Duda, B.A.; Suchman, E.; Jacobs-Lorena, M.; Beaty, B.J.; Carlson, J.O. Aedes aegypti Transducing Densovirus Pathogenesis and Expression in Aedes aegypti and Anopheles gambiae Larvae. Insect Mol. Biol. 2001, 10, 397–405.
- Carlson, J.; Suchman, E.; Buchatsky, L. Densoviruses for Control and Genetic Manipulation of Mosquitoes. Adv. Virus Res. 2006, 68, 361–392.
- Ren, X.; Hoiczyk, E.; Rasgon, J.L. Viral Paratransgenesis in the Malaria Vector Anopheles gambiae. PLoS Pathog. 2008, 4, e1000135.
- Johnson, R.M.; Rasgon, J.L. Densonucleosis Viruses (‘Densoviruses’) for Mosquito and Pathogen Control. Curr. Opin. Insect Sci. 2018, 28, 90–97.
- Riehle, M.A.; Moreira, C.K.; Lampe, D.; Lauzon, C.; Jacobs-Lorena, M. Using Bacteria to Express and Display Anti-Plasmodium Molecules in the Mosquito Midgut. Int. J. Parasitol. 2007, 37, 595–603.
- Favia, G.; Ricci, I.; Marzorati, M.; Negri, I.; Alma, A.; Sacchi, L.; Bandi, C.; Daffonchio, D. Bacteria of the Genus Asaia: A Potential Paratransgenic Weapon against Malaria. Transgenes. Manag. Vector Borne Dis. 2008, 627, 49–59.
- Dehghan, H.; Mosa-Kazemi, S.H.; Yakhchali, B.; Maleki-Ravasan, N.; Vatandoost, H.; Oshaghi, M.A. Evaluation of Anti-Malaria Potency of Wild and Genetically Modified Enterobacter Cloacae Expressing Effector Proteins in Anopheles stephensi. Parasites Vectors 2022, 15, 63.
- Rasgon, J.L. Using Infections to Fight Infections: Paratransgenic Fungi Can Block Malaria Transmission in Mosquitoes. Future Microbiol. 2011, 6, 851–853.
- Tzschaschel, B.D.; Guzmán, C.A.; Timmis, K.N.; Lorenzo, V.d. An Escherichia coli Hemolysin Transport System-Based Vector for the Export of Polypeptides: Export of Shiga-like Toxin IIeB Subunit by Salmonella typhimurium AroA. Nat. Biotechnol. 1996, 14, 765–769.
