A group of metal-containing enzymes named superoxide dismutases (SODs) have vigorous antioxidant roles categorized by their scavenging of ROS [
27]. The SODs have been thought to be the first line of protection arrangement against oxidative stresses, which implies that SODs can play an important defensive role in several cell apoptosis. Before some of ROS can oxidize critical DNA and/or proteins, SODs catalyze the reaction of superoxides to the less damaging/reactive hydrogen peroxide [
28]. The presence of metals such as Cu, Zn, Mn, or Fe may be essential for this function in the system [
29]. So, altered metal homeostasis in cells may be the cause of endogenous oxidative stress [
30]. Three types of SODs are known in mammalian species. The most abundant cytosolic enzyme is SOD1. The loss of SOD1 increases the total level of ROS, which is thought to trigger oxidative DNA damage to cells. It has been shown SOD1-null animals develop some age-related diseases [
31]. Amazingly, SOD1 may upturn the therapeutic potential of MSCs [
32]. SOD2 is located in the mitochondrial matrix [
33], which is the critical site of free radical production from the electron transportation chain. SOD2 is required for maintaining mitochondrial functions and reliability [
34]. One of the primary functions of SOD2 might be to protect mitochondrial DNA against oxidative damage [
35]. The SOD2 gene is subjected to regulation by a number of inflammatory cytokines and growth factors [
36]. It has been shown that SOD2 overexpression causes an increase in ATP production through energetic mitochondrial respiration [
37]. The induction of osteogenesis in MSCs is associated with an upregulation of SOD2 and a decrease in ROS levels [
38]. Additionally, the reduction of SOD2 diminishes the expression of an adipogenesis marker, which results in higher ROS production [
39]. SOD3 is secreted to the extracellular matrix in cells and tissues [
40]. Downregulation of SOD3 has been shown to lead DNA copy number change and/or hypermethylation in the promoter region of genes [
41]. It has been revealed that overexpressed SOD3 causes hypoxic accumulation of hypoxia inducible factor-1α (HIF1α) in cells [
42]. There is an increase in SOD3 expression with the differentiation of MSCs into adipocytes [
43]. In addition, SOD3 levels have been shown to decrease upon chondrogenesis [
43].
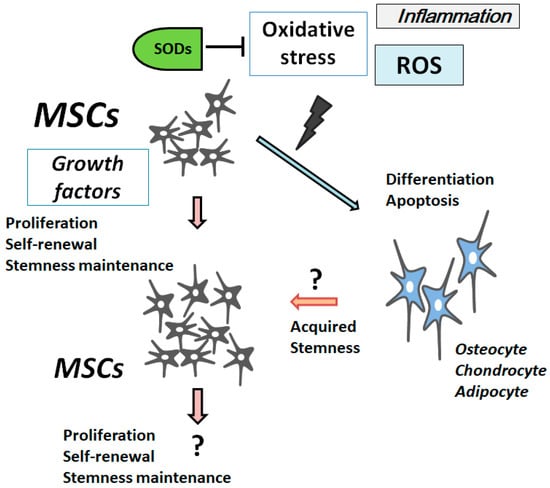
In MSCs, excess ROS can impair self-renewal, differentiation capacity, and proliferation [
44]. Concordantly, antioxidants stimulate MSCs’ proliferation [
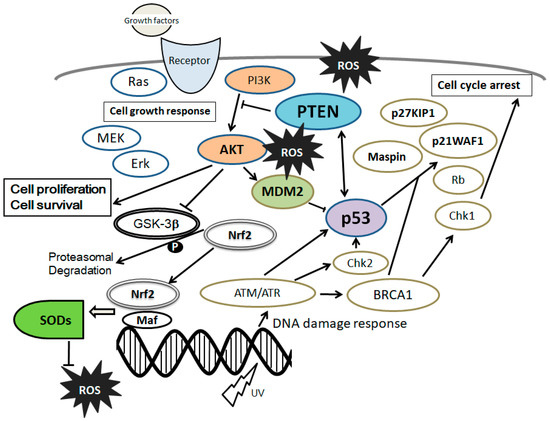
45]. The MSCs’ stemness is maintained by inhibiting cellular senescence through a PI3K/AKT pathway [
46]. Moreover, the PI3K/AKT pathway has been shown to be involved in maintaining embryonic stem cell pluripotency [
47]. Activation of the PI3K/AKT signaling may have dynamic roles in maintaining the pluripotency of stem cells [
48], which is also involved in enhanced cell proliferation [
49] (
Figure 2). In addition, it has been reported that PI3K/AKT is associated with the regulation of stem cell fate [
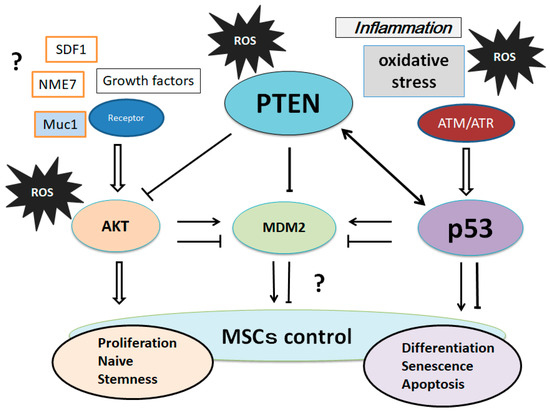
50]. Stromal cell-derived factor 1 (SDF1) is an important chemokine in stem cell mobilization, and plays a critical role in the biological functions of MSCs by enhancing PI3K expression [
51]. Muc1 is a member of the carbohydrate-binding protein family that contributes to MSCs’ survival and stemness via the PI3K/AKT signaling [
52].