Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Metallic trace elements toxicity has been associated with a wide range of morphological abnormalities in fish, both in natural aquatic ecosystems and controlled environments. The bioaccumulation of metallic trace elements can have devastating effects on several aspects of fish health, encompassing physiological, reproductive, behavioural, and developmental functions.
- metallic trace elements
- toxicity
- growth
- fish
- ROS
- contaminated sites
- nervous system
- reproductive system
- embryonic development
1. Introduction
Recent advancements in industrialization and increased human influence on the environment have caused an exponential increment of different pollutants, such as dyes, metallic trace elements, pharmaceuticals, pesticides, fluoride, phenols, insecticides, and detergents which enter into water resources [1]. These toxicants are serious health concerns for humans and water-living organisms [2]. Similarly, surface water contamination by pesticides is also a serious health-related and environmental issue highlighted at different forums [3]. Bioaccumulation of pollutants in an aquatic ecosystem affects humans and marine life directly and indirectly through the food chain [4]. Metallic trace elements like Cd, Co, Ni, and Pb have been found to impact fishes and other aquatic organisms directly [5]. Majority of metallic trace elements also act as environmental toxins. Some of these metallic trace elements, such as Cu, Zn, Cr, Pb, Cd, Hg, and As affect the health of living beings more adversely as these could quickly transfer from one trophic level to another and hence show higher persistence in the food web [6]. Moreover, the ions of trace elements in water bodies have also become a serious concern globally, as these metallic ions have shown adverse effects on the aquatic ecosystem, and human health [7]. Therefore, simple but effective methods are required for their detection and to maintain water quality to solve water scarcity and further its reuse [8][9]. Despite being able to cause serious damage, these metals are not being identified easily due to insufficient methods and limited laboratory facilities. The present detection methods like UV–Visible spectroscopy, atomic emission spectroscopy (AAS), gas chromatography/mass spectrometries (GC/MS) are not economic and user-friendly [10]. For instance, the technological advancements have raised major concern over environmental safety, due to increasing generation of toxicants [11]. To overcome this and provide ease of analysis, with accuracy and cost effectively, “biosensor” came to existence. A biosensor has a readable biological element, responsible for providing and transforming the information that is used to detect the concentration of a particular analyte in environment. The bio-element based sensors are qualitative, quantitative, and semi-quantitative and can be used against conventional methods [12]. Biosensors possess unique features that make them more adept at measuring the level of metallic trace elements concentration on-site and therefore are advantageous in water quality control. For instance, ligand-rich membranes like tannin-reinforced 3-aminopropyltriethoxysilane crosslinked polycaprolactone (PCL) based nanofibrous membrane have shown effective and quick response to trace elements’ toxicity as compared to uncross linked membranes [13].
Figure 1 illustrates the impact of metallic trace elements on fish from different sources. These selected metals belong to the first transition series of the periodic table and are known to trigger the production of reactive oxygen species (ROS) in living systems, which contribute to their toxicity [14][15]. Exposure to sub-lethal or lethal concentrations of metallic trace elements can lead to stress in fish, which eventually accumulates in various tissues and organs such as gills, kidneys, liver, skin, muscles, etc. [16]. Fish have their defence mechanism to cope with the stressful conditions caused by metallic trace elements exposure by utilizing more energy from reserved carbohydrates, proteins, and lipids in their body. Metallic trace elements such as As, Cd, Cr, Cu, Fe, Hg, Ni, Pb, and Zn are active redox components that contribute to the formation of ROS, which play an essential role in certain physiological functions in fish [15].
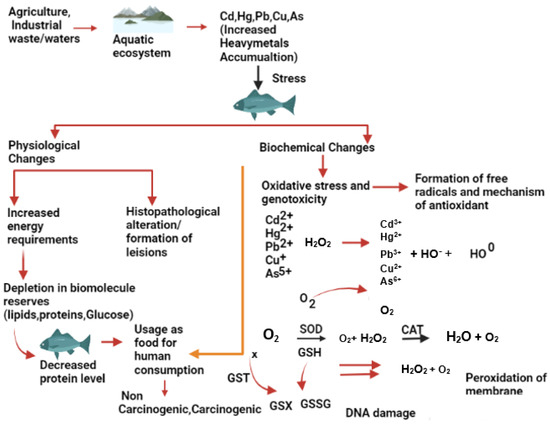
Figure 1. Effects of metallic trace elements on the fish physiology and biochemistry.
The excess of ROS Indicates an imbalance in the production of ROS and causes oxidative stress, which eventually interferes with cellular function by damaging lipids, proteins and DNA [17]. Enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione S transferase (GST), and non-enzymatic compounds such as reduced glutathione (GSH) are essential in maintaining the dynamic balance of ROS through detoxification. SOD converts superoxide free radicals into hydrogen peroxide, which is further broken down into non-toxic oxygen and water by the CAT enzyme [18]. GST aids in detoxification by catalysing the conjugation of electrophiles to GSH. However, electrophilic substances (free radicals and ROS) can also oxidize GSH non-enzymatically to glutathione disulfide. Any hindrance in the enzymatic reaction can generate excess ROS that accumulates in fish tissues, leading to oxidative stress. ROS can degenerate the cell membrane through lipid peroxidation, causing genotoxicity through DNA damage [17]. There is a wealth of information on the effects of metallic trace elements on fish physiology in various fish species.
2. Effect of Metallic Trace Elements on Fish Collected from Contaminated Sites
Estuaries are highly sensitive zones that serve as a natural conduit for transferring agricultural, industrial, and urban pollution to the sea [19]. Rapid industrial growth during the past century has led to an increase in industrial effluents [20] and anthropogenic run-off in coastal and estuarine environments [21]. The fate of metallic trace elements in water is mainly influenced by their initial concentration and several chemical, physical, and biological factors [22]. Table 1 provides details on the effects of metallic trace elements on fish collected from various contaminated sites.
Table 1. Effects of heavy metals on fish collected from different contaminated sites.
| Fish Specie | Location | Metal Detected |
Organ Affected | Effect on Fish | References |
|---|---|---|---|---|---|
| Channa striata, Heteropnuestes fossilis |
Yamuna Barrage (India) | Cr, Ni, Pb | Kidney, gills, liver, muscle |
Ruptured veins, hemorrhages in the liver, necrotic urinary tubules. | [23] |
| Clarias gariepinus | Abuja (Nigeria) | Pb, Cd, Cu, Zn, Cr | Liver, gill, kidney, spleen | Congested central veins in the liver, interstitial hemorrhages in the kidney, congested splenic vein. | [24] |
| Cyprinus carpio | Slovak University of Agriculture in Nitra, University Farm Kolíňany | Cu, As, Pb, Cr, Cd, Hg | Testes | Reduced sperm DNA fragmentation, reduced motility of spermatozoa. | [25] |
| Cyprinus carpio and Capoeta | Kor River (Fars Province) | Hg, Cd, As, Pb | Blood cells, liver, kidney | Hyperemia, cellular degeneration, and vacuolation. | [26] |
| Oreochromis niloticus | Challawa River (Kano, Nigeria) | Zn, Cd, Fe, Pb | Muscles | Higher bioaccumulation in muscles compared to bioaccumulation factor. | [27] |
| Clarias gariepinus | Lake Maryout (Egypt) | Cd, Pb, Hg, As | Gonads | The ovary exhibits lytic characteristics with oocytes at various stages, a decreased quantity of germinal cells, and an augmented interstitial space in the testes. | [28] |
| Auchenoglanis occidentalis | Tiga Dam (Nigeria) | Zn, Cd, Pb, Fe | Gills, liver, kidney | Lesions in the gills, liver, and kidney. | [29] |
| Hypophthalmichthys molitrix, Ctenopharyngodon idellus, Carassius auratus, Cyprinus carpio, Silurus asotus | Yangtze River | Cd, Cr, Cu, Hg, Pb, Zn | Fish size | Positive and negative relationships were observed between fish size and metal concentration. | [30] |
| Channa striatus, Heteropneustes fossilis |
Kali River (India) | Cr, Cd, Pb, Ni | Liver, kidney, gill, muscle, brain | Decreased level of glutathione (GSH), increased oxidative stress. | [31] |
| Etroplus maculates, Cirrhinus reba, and Ompok bimaculatus | Bhadra River (Karnataka) |
Cu, Zn, Cd, Ni, Fe, Pb | Liver, kidney, muscle, gills |
Degeneration of the hepatocytes in liver, vacuolar degeneration in the tubular epithelium in kidney. | [32] |
| Oreochromis niloticus, Geophagus brasiliensis, Hoplias malabaricus, Astyanax altiparanae, Rhamdia quelen | Sao Francisco do Sul River (Brazil) | Cr, Mn, Fe, Ni, Cu, Zn, As, Se, Pb | Muscle, liver, and gonads | Metals accumulated in the gonads, liver, and muscle, with chromium levels in the muscle reaching fifty times the maximum limit set by Brazilian legislation. | [33] |
| Oligosarcus spp., Chyphocharax voga | Sinos River (Brazil) | Al, As, Cd, Co, Cr, Cu, Fe, Mn, Zn, Pb | Liver | Detritivores species accumulated more metals than carnivorous species. | [34] |
| Salminus franciscanus | Paraopeba River (Brazil) | Cu, Pb, Cd, Zn, Cr, Hg, Fe | Liver, spleen, and muscle | Hepatocytes exhibited fat accumulation along with pigmented macrophages in the liver. Fibrosis was observed in the spleen, and contaminated fish showed decreased oocyte diameter and increased follicular atresia. | [35] |
| Pseudoplatystoma corruscans | Paraopeba River (Brazil) | Hg, Cd, Zn, Cr, Pb | Liver, muscle, and spleen | The liver and spleen showed higher concentrations of metals compared to the muscle. Additionally, liver fibrosis was observed. | [36] |
| Bryconamericus iheringii | Ilha River (Brazil) |
Al, Cd, Mn, Ni, Fe, Pb, Cr, Zn | Blood—micronucleus analysis, gills, and muscle | In rural areas, a higher frequency of micronuclei, nuclear abnormalities, and mucous cells was detected. Conversely, urban areas exhibited a lower condition factor, higher frequencies of lamellar alterations, and higher concentrations of chromium (Cr) and nickel (Ni) in muscle. | [37] |
| Prochilodus magdalenae, Pimelodus blochii | Magdalena River (Colombia) | Cd, Pb, Ni | Gills, liver, and muscle | Pimelodus Blochii showed a higher accumulation of metals, particularly an increased concentration of cadmium (Cd) in the liver. | [38] |
| Aequidens metae, Astyanax bimaculatus | Ocoa River (Colombia) |
Hg, Cd | Blood and liver | There was a decrease in the number of erythrocytes, lymphocytes, and neutrophils, as well as a decrease in hemoglobin concentration and hematocrit percentage. | [39] |
3. Effect on the Nervous System
Deposition of various metallic trace elements in fish can cause serious damage to the nervous system, affecting behaviour, response to stimuli, and recognition patterns among fish [40]. Mercury is known to cause numerous disorders, primarily on the biochemical level in the central nervous system of fish. For example, exposure to HgCl caused a significant increase in lipid peroxidation and depletion of total lipids in the brain of catfish (Heteropneustes fossilis) [41]. Copper-induced morphological abrasions are evident in the sensory organs of fish [40]. Copper is a vital metal and a fundamental component of many enzymes, but it can be extremely toxic to fish when its concentration exceeds normal levels [42], especially in freshwater due to the high ionic copper content [43]. Increased Cu concentration in cellular membranes reduces the antioxidative capacity of lipids, causing lipid peroxidation and severe damage to cellular membranes [44].
As the formation of free radicals and lipid peroxidation increases, they can cause serious cellular trauma. In Cu-exposed marbled electric ray (Torpedo marmorata), ultrastructural analysis of neurons in the central nervous system showed an increased number of lipofuscin granules erosion of mitochondria [45] and a reduction in Golgi apparatus as well [46]. Long-term exposure to Pb can cause neurochemical changes in the brain of walking catfish (Clarias bathrachus). For instance, Pb increases the histamine and serotonin levels while decreasing the gamma-aminobutyric acid (GABA), monoamine oxidase (MAO), and acetylcholinesterase (AChE) contents. Furthermore, cholesterol, brain lipid, and protein contents are also decreased [47]. The adverse effects of different metals on the nervous system (CNS and peripheral) from various studies are compared (Table 2).
Table 2. Effects of heavy metals on nervous system of different fish species.
| Fish Species | Metal Concentration (mg L−1) | Metal Composition | Stage of Exposure | Exposure Duration | Effect Observed on Fish | References |
|---|---|---|---|---|---|---|
| Effect of Cadmium (Cd) | ||||||
| Danio rerio | 0.970 | Cd † | Juvenile | 12 h | Elevated immunotoxicology. | [48] |
| Danio rerio | 0.040 | CdCl2 | 0–168 hpf | 7 days | Increased rotational movement, Hyperactivity, and decreased size of otolith. | [49] |
| Pimephales promelas | 0.003 | Cd(NO3)2 | Adult | 4 days | Elevated auditory threshold. | [50] |
| Pimephales promelas | 0.060 | CdCl2 | Adult | 21 days | Decreased vitellogenin gene expression and increased estrogen receptor beta. | [51] |
| Danio rerio | 0.112 | CdCl2 | 0–96 hpf | 4 days | Immunotoxicity, behavioural alteration, and oxidative stress. | [52] |
| Effect of Mercury (Hg) | ||||||
| Diplodus sargus | 0.002 | HgCl2 | Juvenile | 7 days | Increased anxiety, decreased number of optic tectum cells, and altered swim behaviour. | [53] |
| Pimephales promelas | 0.720 | MeHg | Adult | 30 days | Decreased levels of dopamine and hyperactivity. | [54] |
| Danio rerio | 10 | MeHg | Adult | 56 days | Mitochondrial dysfunction, and oxidative phosphorylation. |
[55] |
| Danio rerio | 0.720 | MeHg | Adult and embryo | 30 days | Decreased level of dopamine and hyperactivity. | [56] |
| Danio rerio | 0.027 | HgCl2 | 5–72 hpf | ~3 days | Hyperactivity causing mortality. | [57] |
| Effect of Lead (Pb) | ||||||
| Danio rerio | 0.010 | Pb(CH3COO)2 | 0–72 hpf | 3 days | 10 Gene expression changes in 89 genes associated with nervous system development. | [58] |
| Danio rerio | 0.020 | Pb(CH3COO)2 | 0–144 hpf | 6 days | Decreased axon length and decreased locomotion (speed). | [59] |
| Danio rerio | 0.100 | Pb(CH3COO)2 | 2–120 hpf | ~5 days | Altered color preference (adults). | [60] |
| Danio rerio | 0.207 | Pb(CH3COO)2 | 2–24 hpf | ~2 days | Decreased learning (adults). | [61] |
| Danio rerio | 1.730 | Pb(CH3COO)2 | 0–24 hpf | 24 h | Decreased Nrxn2a gene expression. | [62] |
| Effect of Copper (Cu) | ||||||
| Cyprinus carpio | 0.60 | Cu | Juvenile | 96 h | Increases in brain ROS production, lipid peroxidation, and protein oxidation. | [63] |
| Capoeta umbla | 3.0 | CuSO4∙5H2O | 112 ± 5 g | 96 h | Induce astroglial response accompanied by modulations of NF-kB and PARP-1 expression. | [64] |
| Danio rerio | 0.100 | CuSO4∙5H2O | Adult | 10 days | Negatively affect the associative learning capabilities. | [65] |
| Oreochromis niloticus | 120 | CuSO4∙5H2O | Adult | 96 h | Loss of balance and exhaustion. | [66] |
| Effect of Arsenic (As) | ||||||
| Danio rerio | 15 | Na2HAsO4 | Adult | 96 h | Alteration in behaviour and ectonucleotidase activities. | [67] |
| Danio rerio | 0.050 | As2O3 | Juvenile | 96 h | Antagonistic effects on brain. | [68] |
| Danio rerio | 0.500 | As+ | Larvae, juvenile and adult | 96 h | Alteration in motor function (embryo-adult), effects on associative learning. |
[69] |
| Clarias batrachus | 20 | As2O3 | Adult | 96 h | Increased body discoloration, excessive mucous secretion, loosening of the skin, and complete loss of skin (head region and fins). | [70] |
| Effect of Zinc (Zn) | ||||||
| Anguilla anguilla | 0.12 | Zn | Juvenile | 28 days | Cholinergic neurotoxicity did not occurr, only liver GST increased significantly. | [71] |
| Leporinus obtusidens | 4.57 | ZnSO4·5H2O | Adult | 45 days | Significantly increased AChE activity. | [72] |
| Danio rerio | 1750 | ZnCl2 | Adult | 25 days | Significant decrease in acetylcholinesterase activity and abnormal neural signaling. | [73] |
Note: † Metallic trace elements written without their respective chemical formulas were administered in their metallic forms.
4. Effect on the Reproductive System
The adverse effects of metals on the fish reproductive system are increasing every day, mainly due to increased water pollution and the usage of polluted water for fish culture. Healthy eggs and sperms are essential for the process of successful fertilization. However, the quality of eggs and sperm is affected by induced spawning, gamete storage methods, and more importantly, water pollution. The motility time of spermatozoa is very important for effective fertilization. According to the literature, sperm motility is affected by metallic trace elements. For example, although the sperm morphology of mummichog (Fundulus heteroclitus) was not affected by methylmercury (CH3Hg), it triggered a significant loss in the motility of sperms [74][75]. Lead, Cd, and Cu caused a significant decrease in the motility of European carp (Cyprinus carpio) spermatozoa [76][77][78].
Similarly, Cu toxicity caused adverse effects in the spermatozoa activity in C. carpio [79], while Sionkowski et al. [80] showed that the higher concentration of Cu and Pb caused reduced spermatozoa motility in grass carp (Ctenopharyngodon idella). Likewise, the effects of Zn on the sperm motility of some common carp were also explored. Metallic trace elements are also responsible for several endocrine complications among fish. For example, Cd decreased the thyroid hormone level, inhibited the estrogen receptors, and interrupted the expression of growth hormone [81]. On the other hand, iodine metabolism interruption by Pb was also recorded to inhibit thyroid synthesis [82]. Prooxidative possessions of the metal ions could also cause oxidative harm to the cell membrane. They can also induce oxidative stress in fish. Lead, Pb, and Cu can also trigger the genotoxic effects on the fish [83][84][85]. A tabulated review of different references is provided to show the deteriorating effects of different metals on fish’s reproductive system (Table 3).
Table 3. Effects of heavy metals on reproductive system of different fish species.
| Fish Species | Metal Concentration (mg L−1) | Metal Composition | Stage of Exposure | Exposure Duration | Effect Observed on Fish | References |
|---|---|---|---|---|---|---|
| Effect of Cadmium (Cd) | ||||||
| Heteropneustes fossilis | 0.050 | CdCl2 | Adult | 24 h | Decreased ovulation. | [86] |
| Pimephales promelas | 0.005 | CdCl2 | 12 months | 21 days | Reduced egg production. | [51] |
| Oryzias melastigma | 0.010 | CdCl2 | 5 months | 30 days | Decreased gonadal development. | [87] |
| Prochilodus magdalenae | 24.90 | CdCl2 | 2 years | 7 days | Reduced fertility rate. | [88] |
| Effect of Mercury (Hg) | ||||||
| Heteropneustes fossilis | 0.050 | HgCl2 | Adult | 24 h | Increased germinal vesicle breakdown. | [86] |
| Cyprinus carpio | 4.990 | HgCl2 | 3 years | 12 h | Decreased motility and fertility of sperms, damaged eggs. | [89] |
| Oncorhynchus mykiss | 10.00 | HgCl2 | 3 years | 4 h | Reduced motility of sperm. | [90] |
| Danio rerio | 0.015 | HgCl2 | Adult | 5 days | Delayed gonadal development, imbalanced sex hormone. |
[91] |
| Danio rerio | 0.030 | HgCl2 | Adult | 30 days | Decreased testosterone level. | [92] |
| Clarias gariepinus | 0.119 | HgCl2 | Adult | 30 days | Disruptive effect on gamete development. | [93] |
| Effect of Lead (Pb) | ||||||
| Heteropneustes fossilis | 0.050 | (Pb(NO3)2 | Adult | 96 h | Increased germinal vesicle breakdown. | [86] |
| Clarias gariepinus | 140.0 | Pb(C2H3O2)2 | Adult | 96 days | Reduced sperm motility. | [94] |
| Oryzias melastigma | 0.050 | PbCl2 | 5 months | 30 days | Decreased gonadal development. | [87] |
| Effect of Copper (Cu) | ||||||
| Danio rerio | 0.040 | CuSO4 | Adult | 30 days | Damaged structure of gonads, altered steroid hormone level. | [95] |
| Pimephales promelas | 0.075 | CuCl2 | 12 months | 21 days | Decreased abundance of post-vitellogenic follicles, increased follicular atresia. | [96] |
| Daphnia magna | 1.041 | CuCl2 | Adult | 21 days | Reduced rate of reproduction. | [97] |
| Poecilia reticulata | 45 | CuO | Adult Larvae |
96 h | Decreased reproduction success. | [98] |
| Poecilia reticulate | 0.026 | CuSO4 5H2O | 2.5–3 months | 56 days | Gonadosomatic index, offspring production decreased. | [99] |
| Effect of Arsenic | ||||||
| Gobiocypris rarus | 40.00 | NaAsO2 | 3 months | 96 days | Accumulation in testis. | [100] |
| Daphnia magna | 0.049 | NaAsO2 | Adult | 48 h | Stable reproduction rate. | [101] |
| Gambusia affinis | 0.075 | NaAsO2 | Juvenile | 30 days | Lower gonadal-somatic indices. | [102] |
| Effect of Zinc (Zn) | ||||||
| Odontesthes bonariensis | 0.021 | ZnSO4 7H2O | Adult | 10 days | Reduced embryo and larval survivability. | [103] |
| Danio rerio | 500 | Zn | Adult | 4 days | Majority of eggs were dead, larger hatching time. | [104] |
| Clarias magur | 300 | Zn(CH3COO)2 | Mature | 60 days | The highest GSI and fecundity. | [105] |
| Oryzias melastigma | 0.010 | ZnSO4·7H2O | Adult | 30 | Irregular oocytes, partly adhesion, empty follicle, and increased follicular atresia, loose follicular lining. | [87] |
5. Effect on Embryonic Development
The influence of water-borne metals can disrupt the embryonic development of spawners. [106] found elevated levels of Cd, Zn, and Pb in the female gonads of stone loach when exposed to toxic concentrations of these metals. Ellenberger et al. [107] investigated the levels of Cu in the reproductive organs of European perch (Perca fluviatilis) exposed to Cu-polluted ponds. White suckers in polluted lakes exhibited higher amounts of Cu and Zn in their testicles and female gonads compared to fish in uncontaminated water [108][109]. Common carp exposed to Cu, Cd, and Pb showed decreased egg swellings in a concentration-dependent manner, contrasting with about 40% expansion in egg width observed in the untreated groups [110]. Copper and Cd accumulation in the gonads of Mozambique tilapia (Orechromis mossambicus) was found to be elevated when fish were kept in metal-polluted water, and blue tilapia (Oreochromis aureus) exposed to Cd and Pb for seven days showed metal accumulation in the testicles and female gonads, particularly Cd levels in the ovaries [111]. Metal exposure to spawners can result in the deposition of metallic trace elements accumulated in eggs and sperm, severely affecting the survival of fertilized eggs and the embryonic development of fish [79].
Metals can also influence the physical characteristics of an egg’s outer surface. Benoit and Holcombe [112] observed that eggs of Zn-exposed fathead minnow (Pimephales promelas) became sticky and more prone to breakage soon after egg laying. Fathead minnow embryos rapidly absorb Hg from surrounding water sources, with concentrations in juveniles increasing to 2.80 µg per gram humid mass after four days of exposure to 25 µg per cubic decimeter of methylmercury [113]. Chromium was found to accumulate in the outer protective coatings of Cyprinus carpio eggs at pH 6.3 [114]. Copper can alter selective membrane permeability, disrupting cation trade between the liquid in the yolk membrane and the outside water [115]. During the early development of fish eggs in a toxic (metallic trace elements) environment, the outer protective coating of the egg blocks most of the metal concentration, but a significant toxic amount still enters the fluid inside the egg membranes, while only a small amount infiltrates the embryo [116]. Beattie and Pascoe [117] found that eggs of Atlantic salmon (Salmo salar) exposed to 10 mg per litre of Cd at 22 h old retained 98% of the metal in the outermost membrane. Similarly, the outer membrane of Japanese rice fish (Oryzias latipes) eggs retained 94.4% of Cd [118]. In Zn-treated Atlantic herring (Clupea harengus) eggs, 30% to 50% of Zn accumulated in the outermost membrane, while the rest accumulated primarily in the yolk sac and in lower quantities in the embryos. However, even a small amount of metals penetrating the egg can significantly influence fish embryonic growth [117][119]. Devlin [113] observed significant abnormalities in fathead minnow embryos treated with Hg, including spinal curves, heart damage, and abnormal growth of the heart cavity. Samson and Shenker [120] reported tissue anomalies in zebrafish (Danio rerio), including abnormalities in fin overlaps and caudal parts.
The first 24 h of fish embryonic development are the most vulnerable to metallic trace elements toxicity. A study found that during the first 24 h after insemination in contaminated water, almost 20% of developing embryos died, even in a controlled environment [121]. The blastula stage had the highest mortality rate (15%), and metal exposure during this stage significantly affected the life span of developing embryos. Embryos exposed to 0.1 mg/L of Cu had significantly decreased survival rates compared to the control group, and at 0.3 mg per litre, all embryos died. Copper exposure caused most fish embryo deaths during the blastula stage (25%), followed by the stage of body division (15%). However, embryo mortality decreased significantly at later developmental stages [79]. Slominski et al. [121] also reported that mortality significantly declined during organ formation, the division of the body, and eye coloration phases. Most fetuses (5%) expired during organ formation before the eye coloration phase. Metallic trace elements toxicity also increases the death of fish hatchlings in various species, including rainbow trout (Onchorynchus mykiss), Atlantic salmon, common carp, and grass carp. Freshly inseminated ovum of Oncorhynchus mykiss were more susceptible to Ni than the embryo at the organogenesis stage, while goldfish (Carassius auratus) eggs’ mortality was greater during the blastula stage than at the eyed stage when exposed to Cd or Hg. Rainbow trout (Oncorhynchus mykiss) fetuses were more vulnerable at the eyed stage than newly inseminated eggs when presented with a mixture of metals. The abnormalities caused by different metallic trace elements at the embryonic stages of fish are reviewed in Table 4.
Table 4. Effects of heavy metals on embryonic development fish species.
| Fish Species | Metal Concentration (mg L−1) | Metal Composition | Stage of Exposure | Exposure Duration | Effect Observed on Fish | References |
|---|---|---|---|---|---|---|
| Effect of Cadmium (Cd) | ||||||
| Leuciscus idus | 0.1000 | CdCl2 | Egg, sperm | 21 dpf | Reduced larval survival, growth, and delayed development. | [78] |
| Oryzias latipes | 0.0019 | CdCl2 2H2O | Embryo, larva | 20 dpf | Morphological abnormalities were observed. | [122] |
| Cyprinus carpio | 0.06 | CdCl2 | Eggs | 60 dpf | Retardation in the developmental stages of eye pigmentation and spine curvature, lack of tail formation and head. | [123] |
| Danio rerio | 34.8 | CdCl2 | 72 hpf | 72 h | Neuromast damage, coagulated egg, increased mortality rate. | [124] |
| Danio rerio | 0.8018 | CdCl2 | 6 hpf | 24 h | Increased apoptotic event and induced cell death in brain of embryo. | [125] |
| Leuciscus idus | 0.1 | CdCl2 | Embryonic and larval | 21 days | Reduced embryonic survival, increased frequency of malformation, and delayed hatching. | [78] |
| Danio rerio | 0.8909 | CdCl2 | Embryonic and larval | 96 hpf | Increased heartbeat rate of larvae and decreased brain size. | [126] |
| Leuciscus idus L. | 0.1 | CdCl2 | Embryos and newly hatched larvae | 2 h | Reduced egg swelling, slowed the rate of development (especially body movements), and delayed hatching. | [127] |
| Odontesthes bonariensis | 0.00025 | CdCl2 | Advanced-stage embryos and newly hatched larvae | 10 days | Decreased hatching rate and survival of embryo and larvae. | [103] |
| Effect of Mercury (Hg) | ||||||
| Danio rerio | 0.016 | HgCl2 | Adult | 2 hpf | T3 and T4 content in larvae increased. | [128] |
| Danio rerio | 0.016 | HgCl2 | Adult | 168 hpf | Decreased hatching rate, increased mortality, increased malformation rate in larvae. | [92] |
| Cyprinus carpio | 0.00001 | HgCl2 | Embryo | 96 h | SOD and GPx reduced up to 85%. | [129] |
| Effect of Lead (Pb) | ||||||
| Danio rerio | 0.100 | Pb (C2H3O2)2 | Adult | 30 dpf | Distance moved by juvenile zebra fish decreased, and swimming activity alterations in larvae and juvenile fish. | [130] |
| Danio rerio | 0.005 | Pb (CH3COO)2 | Adult | 144 hpf | Delayed hatching, spinal and tail deformity, pericardial edema, and yolk swelling was observed. | [131] |
| Danio rerio | 99.885 | Pb (C2H3O2)2 | Adult | 72 hpf | Deformed CNS, increased levels of Gamma-aminobutyric acid (primary inhibitory neurotransmitter). | [132] |
| Danio rerio | 1.6 | Pb (NO₃)₂ | Embryo | 120 hpf | Spinal malformation. | [133] |
| Pterophyllum scalar | 20 | PbCl2 | Embryo | 3 days | Tilt, loss of vision or the lack of effect on growth delay. | [134] |
| Effect of Copper (Cu) | ||||||
| Leuciscus idus | 0.100 | CuSO4·5H2O | Egg, sperm | 21 dpf | Reduced larval survival, growth, and delayed development. | [78] |
| Oryzias latipes | 0.0185 | CuCl2 H2O | Embryo, larva | 20 dpf | Percentage of deformed larvae significantly increased. | [122] |
| Poecilia reticulata | 1.50 | CuSO4·5H2O | Embryo | 15 days | Abnormalities in blastodisc to middle-eyed stages of development. | [135] |
| Danio rerio | 0.018 | CuSO4 | 72 hpf | 72 h | Neuromast damage, coagulated egg, increased mortality rate. | [124] |
| Leuciscus idus | 0.10 | CuSO4·5H2O | Embryo and larval | 21 days | Reduced embryonic survival, increased frequency of malformation. | [78] |
| Leuciscus idus L. | 0.10 | CuSO4 | Embryos and newly hatched larvae | 2 h | Reduced egg swelling slowed the rate of development (especially body movements) and delayed hatching. | [127] |
| Odontesthes bonariensis | 0.00025 | CuSO4 | Advanced-stage embryos and newly hatched larvae | 10 days | Decreased hatching rate and survival of embryo and larvae. | [103] |
| Carassius auratus | 1 | Cu2− | Embryo | 24 h post-hatching | Scoliosis and tail curvatures. | [136] |
| Effect of Arsenic (As) | ||||||
| Danio rerio | 360.32 | NaAsO2 | Adult | 120 h | Tail bud deformation in embryo. | [137] |
| Danio rerio | 0.5 | NaAsO2 | Adult | 120 hpf | No effect on mortality and developmental deformations. | [138] |
| Labeo rohita | 198.18 | NaAsO2 | Adult | 120 hpf | Reduced survival rate with abnormal development. | [139] |
| Danio rerio | 0.5 | NaAsO2 | Embryo | 14 dpf | Thinning of the retinal pigmented epithelium (RPE) layer in embryos. | [140] |
| Effect of Zinc (Zn) | ||||||
| Odontesthes bonariensis | 0.021 | ZnSO4 7H2O | Hatchling | 10 days | Cumulative embryo survival was significantly reduced. | [103] |
| Pagrus major | 2.5 | ZnCl2 | 2 years | 10 days | Low hatching rate, high mortality, abnormal pigmentation, hooked tail, spinal deformity, pericardial edema, and visceral hemorrhage. | [141] |
| Melanotaenia fluviatilis | 33.3 | Zn | Embryo | 2 h | Spinal deformities. | [142] |
This entry is adapted from the peer-reviewed paper 10.3390/w15163017
References
- Reddy, D.H.; Lee, S.-M. Water Pollution and Treatment Technologies. J. Environ. Anal. Toxicol. 2012, 2, e103.
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077.
- Vieira, C.E.; Costa, P.G.; Lunardelli, B.; De Oliveira, L.F.; Cabrera Lda, C.; Risso, W.E.; Primel, E.G.; Meletti, P.C.; Fillmann, G.; Martinez, C.B. Multiple biomarker responses in Prochilodus lineatus subjected to short-term in situ exposure to streams from agricultural areas in Southern Brazil. Sci. Total Environ. 2016, 542, 44–56.
- Naqvi, G.; Shoaib, N.; Majid, A. Genotoxic potential of pesticides in the peripheral blood erythrocytes of fish (Oreochromis mossambicus). Pak. J. Zool. 2016, 48, 1643–1648.
- Ezemonye, L.I.; Adebayo, P.O.; Enuneku, A.A.; Tongo, I.; Ogbomida, E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol. Rep. 2019, 6, 1–9.
- Pujari, M.; Kapoor, D. Heavy metals in the ecosystem: Sources and their effects. In Heavy Metals in the Environment; Kumar, V., Sharma, A., Cerdà, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021.
- Ullah, S.; Zahra, Q.U.A.; Mansoorianfar, M.; Hussain, Z.; Ullah, I.; Li, W.; Kamya, E.; Mehmood, S.; Pei, R.; Wang, J. Heavy Metal Ions Detection Using Nanomaterials-Based Aptasensors. Crit. Rev. Anal. Chem. 2022, 1–17.
- Liu, Y.; Wang, P.; Gojenko, B.; Yu, J.; Wei, L.; Luo, D.; Xiao, T. A review of water pollution arising from agriculture and mining activities in Central Asia: Facts, causes and effects. Environ. Pollut. 2021, 291, 118209.
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Yegya Raman, P.K.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2022, 287, 132187.
- Ramnani, P.; Saucedo, N.M.; Mulchandani, A. Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants. Chemosphere 2016, 143, 85–98.
- Tripathi, S.; Poluri, K.M. Heavy metal detoxification mechanisms by microalgae: Insights from transcriptomics analysis. Environ. Pollut. 2021, 285, 117443.
- Hassani, S.; Rezaei Akmal, M.; Salek Maghsoudi, A.; Rahmani, S.; Vakhshiteh, F.; Norouzi, P.; Ganjali, M.R.; Abdollahi, M. High-performance voltammetric aptasensing platform for ultrasensitive detection of bisphenol A as an environmental pollutant. Front. Bioeng. Biotechnol. 2020, 8, 574846.
- Hussain, Z.; Ullah, S.; Yan, J.; Wang, Z.; Ullah, I.; Ahmad, Z.; Zhang, Y.; Cao, Y.; Wang, L.; Mansoorianfar, M.; et al. Electrospun tannin-rich nanofibrous solid-state membrane for wastewater environmental monitoring and remediation. Chemosphere 2022, 307, 135810.
- Mohamed, A.A.-R.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.M.; Ahmed, A.I.; El-Hakim, Y.M.A. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890.
- Naz, S.; Mansouri, B.; Chatha, A.M.M.; Ullah, Q.; Abadeen, Z.U.; Khan, M.Z.; Khan, A.; Saeed, S.; Bhat, R.A. Water quality and health risk assessment of trace elements in surface water at Punjnad Headworks, Punjab, Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 61457–61469.
- Benjamin, L.V.; Kutty, R. Sub-lethal effects of potassium dichromate on hematological and histological parameters in climbing perch, Anabas testudineus (Anabantidae). Int. J. Aquat. Biol. 2019, 7, 140–145.
- Javed, M.; Ahmad, M.I.; Usmani, N.; Ahmad, M. Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci. Rep. 2017, 7, 1675.
- Vutukuru, S.S.; Prabhath, N.A.; Raghavender, M.; Yerramilli, A. Effect of Arsenic and Chromium on the Serum Amino-Transferases Activity in Indian Major Carp, Labeo rohita. Int. J. Environ. Res. Public Health 2007, 4, 224–227.
- Roast, S.D.; Widdows, J.; Jones, M.B. Effects of salinity and chemical speciation on cadmium accumulation and toxicity to two mysid species. Environ. Toxicol. Chem. 2001, 20, 1078–1084.
- Khurana, M.; Nayyar, V.; Bansal, R.; Singh, M. Heavy metal pollution in soils and plants through untreated sewage water. In Ground Water Pollution, Proceedings of the International Conference on Water and Environment (WE-2003), Bhopal, India, 15–18 December 2003; Allied Publishers: New Delhi, India, 2003; pp. 487–495.
- Peng, X.; Zhang, G.; Mai, B.; Hu, J.; Li, K.; Wang, Z. Tracing anthropogenic contamination in the Pearl River estuarine and marine environment of South China Sea using sterols and other organic molecular markers. Mar. Pollut. Bull. 2005, 50, 856–865.
- Katsoyiannis, I.A.; Zouboulis, A.I. Application of biological processes for the removal of arsenic from groundwaters. Water Res. 2004, 38, 17–26.
- Fatima, M.; Usmani, N. Histopathology and bioaccumulation of heavy metals (Cr, Ni and Pb) in fish (Channa striatus and Heteropneustes fossilis) tissue: A study for toxicity and ecological impacts. Pak. J. Biol. Sci. 2013, 16, 412–420.
- Abalaka, S.E.; Enem, S.I.; Idoko, I.S.; Sani, N.A.; Tenuche, O.Z.; Ejeh, S.A.; Sambo, W.K. Heavy metals bioaccumulation and health risks with associated histopathological changes in Clarias gariepinus from the kado fish market, abuja, nigeria. J. Health Pollut. 2020, 10, 200602.
- Kovacik, A.; Tirpak, F.; Tomka, M.; Miskeje, M.; Tvrda, E.; Arvay, J.; Andreji, J.; Slanina, T.; Gabor, M.; Hleba, L.; et al. Trace elements content in semen and their interactions with sperm quality and RedOx status in freshwater fish Cyprinus carpio: A correlation study. J. Trace Elem. Med. Biol. 2018, 50, 399–407.
- Ebrahimi, M.; Taherianfard, M. The effects of heavy metals exposure on reproductive systems of cyprinid fish from Kor River. Iran. J. Fish. Sci. 2011, 10, 13–24.
- Sani, A.; Idris, K.M.; Abdullahi, B.A.; Darma, A.I. Bioaccumulation and health risks of heavy metals in Oreochromis niloticus, sediment and water of Challawa river, Kano, Northwestern Nigeria. Environ. Adv. 2022, 7, 100172.
- HAbdel-Kader, H.H.; Mourad, M. Bioaccumulation of heavy metals and physiological/histological changes in gonads of catfish (Clarias gariepinus) inhabiting Lake Maryout, Alexandria, Egypt. Egypt. J. Aquat. Biol. Fish. 2019, 23, 363–377.
- Abalaka, S.E. Heavy metals bioaccumulation and histopathological changes in Auchenoglanis occidentalis fish from Tiga dam, Nigeria. J. Environ. Health Sci. Eng. 2015, 13, 67.
- Fatima, M.; Usmani, N.; Firdaus, F.; Zafeer, M.F.; Ahmad, S.; Akhtar, K.; Husain, S.D.; Ahmad, M.H.; Anis, E.; Hossain, M.M. In vivo induction of antioxidant response and oxidative stress associated with genotoxicity and histopathological alteration in two commercial fish species due to heavy metals exposure in northern India (Kali) river. Comp. Biochem. 2015, 176, 17–30.
- Yi, Y.J.; Zhang, S.H. Heavy metals (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ. Sci. Pollut. Res. 2012, 19, 3989–3996.
- Shivakumar, C.; Thippeswamy, B.; Tejaswikumar, M.; Prashanthakumara, S. Bioaccumulation of heavy metals and its effect on organs of edible fishes located in Bhadra River, Karnataka. Int. J. Res. Fish. Aquac. 2014, 4, 90–98.
- Espinoza-Quiñones, F.R.; Módenes, A.N.; Palácio, S.M.; Szymanski, N.; Welter, R.A.; Rizzutto, M.A.; Borba, C.E.; Kroumov, A.D. Evaluation of trace element levels in muscles, liver and gonad of fish species from São Francisco River of the Paraná Brazilian state by using SR-TXRF technique. Appl. Radiat. Isot. 2010, 68, 2202–2207.
- Weber, P.; Behr, E.R.; Knorr, C.D.L.; Vendruscolo, D.S.; Flores, E.M.; Dressler, V.L.; Baldisserotto, B. Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem. J. 2013, 106, 61–66.
- Savassi, L.A.; Paschoalini, A.L.; Arantes, F.P.; Rizzo, E.; Bazzoli, N. Heavy metal contamination in a highly consumed Brazilian fish: Immunohistochemical and histopathological assessments. Environ. Monit. Assess. 2020, 192, 542.
- Arantes, F.P.; Savassi, L.A.; Santos, H.B.; Gomes, M.V.; Bazzoli, N. Bioaccumulation of mercury, cadmium, zinc, chromium, and lead in muscle, liver, and spleen tissues of a large commercially valuable catfish species from Brazil. An. Acad. Bras. Cienc. 2016, 88, 137–147.
- Dalzochio, T.; Ressel Simões, L.A.; Santos De Souza, M.; Prado Rodrigues, G.Z.; Petry, I.E.; Andriguetti, N.B.; Herbert Silva, G.J.; Gehlen, G.; Basso Da Silva, L. Water quality parameters, biomarkers and metal bioaccumulation in native fish captured in the Ilha River, southern Brazil. Chemosphere 2017, 189, 609–618.
- Noreña-Ramirez, D.A.; Murillo-Perea, E.; Guio-Duque, A.J.; Méndez-Arteaga, J.J. Heavy metals (Cd, Pb and Ni) in fish species commercially important from Magdalena river, Tolima tract, Colombia. Rev. Tumbaga 2012, 2, 61–76.
- Corredor-Santamaría, W.; Serrano Gómez, M.; Velasco-Santamaría, Y.M. Using genotoxic and haematological biomarkers as an evidence of environmental contamination in the Ocoa River native fish, Villavicencio-Meta, Colombia. Springerplus 2016, 5, 351.
- Baatrup, E. Structural and functional effects of Heavy metals on the nervous system, including sense organs, of fish. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 100, 253–257.
- Bano, Y.; Hasan, M. Mercury induced time-dependent alterations in lipid profiles and lipid peroxidation in different body organs of catfish Heteropneustes fossilis. J. Environ. Sci. Health Part B 1989, 24, 145–166.
- Brown, V.M.; Dalton, R.A. The acute lethal toxicity to rainbow trout of mixtures of copper, phenol, zinc and nickel. J. Fish Biol. 1970, 2, 211–216.
- Miller, T.G.; Mackay, W.C. The effects of hardness, alkalinity and pH of test water on the toxicity of copper to rainbow trout (Salmo gairdneri). Water Res. 1980, 14, 129–133.
- Kumar, K.S.; Rowse, C.; Hochstein, P. Copper-induced generation of superoxide in human red cell membrane. Biochem. Biophys. Res. Commun. 1978, 83, 587–592.
- Aloj Totaro, E.; Pisanti, F.A.; Glees, P.; Continillo, A. The effect of copper pollution on mitochondrial degeneration. Mar. Environ. Res. 1986, 18, 245–253.
- Enesco, H.E.; Pisanti, F.A.; Aloj Totaro, E. The effect of copper on the ultrastructure of Torpedo marmorata neurons. Mar. Pollut. Bull. 1989, 20, 232–235.
- Katti, S.R.; Sathyanesan, A.G. Lead nitrate induced changes in the brain constituents of the freshwater fish Clarias batrachus. Neurotoxicology 1986, 7, 47–51.
- Zheng, J.-L.; Yuan, S.-S.; Wu, C.-W.; Lv, Z.-M.; Zhu, A.-Y. Circadian time-dependent antioxidant and inflammatory responses to acute cadmium exposure in the brain of zebrafish. Aquat. Toxicol. 2017, 182, 113–119.
- Green, A.J.; Mattingly, C.J.; Planchart, A. Cadmium Disrupts Vestibular Function by Interfering with Otolith Formation. bioRxiv 2017.
- Low, J.; Higgs, D.M. Sublethal effects of cadmium on auditory structure and function in fathead minnows (Pimephales promelas). Fish Physiol. Biochem. 2015, 41, 357–369.
- Driessnack, M.K.; Matthews, A.L.; Raine, J.C.; Niyogi, S. Interactive effects of chronic waterborne copper and cadmium exposure on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 165–173.
- Ruiter, S.; Sippel, J.; Bouwmeester, M.C.; Lommelaars, T.; Beekhof, P.; Hodemaekers, H.M.; Bakker, F.; Van Den Brandhof, E.-J.; Pennings, J.L.A.; Van Der Ven, L.T.M. Programmed Effects in Neurobehavior and Antioxidative Physiology in Zebrafish Embryonically Exposed to Cadmium: Observations and Hypothesized Adverse Outcome Pathway Framework. Int. J. Mol. Sci. 2016, 17, 1830.
- Pereira, P.; Puga, S.; Cardoso, V.; Pinto-Ribeiro, F.; Raimundo, J.; Barata, M.; Pousão-Ferreira, P.; Pacheco, M.; Almeida, A. Inorganic mercury accumulation in brain following waterborne exposure elicits a deficit on the number of brain cells and impairs swimming behavior in fish (white seabream—Diplodus sargus). Aquat. Toxicol. 2016, 170, 400–412.
- Bridges, K.; Venables, B.; Roberts, A. Effects of dietary methylmercury on th99e dopaminergic system of adult fathead minnows and their offspring. Environ. Toxicol. Chem. 2017, 36, 1077–1084.
- Rasinger, J.D.; Lundebye, A.-K.; Penglase, S.J.; Ellingsen, S.; Amlund, H. Methylmercury Induced Neurotoxicity and the Influence of Selenium in the Brains of Adult Zebrafish (Danio rerio). Int. J. Mol. Sci. 2017, 18, 725.
- Cambier, S.; Gonzalez, P.; Mesmer-Dudons, N.; Brethes, D.; Fujimura, M.; Bourdineaud, J.-P. Effects of dietary methylmercury on the zebrafish brain: Histological, mitochondrial, and gene transcription analyses. Biometals 2012, 25, 165–180.
- Abu Bakar, N.; Mohd Sata, N.S.; Ramlan, N.F.; Wan Ibrahim, W.N.; Zulkifli, S.Z.; Che Abdullah, C.A.; Ahmad, S.; Amal, M.N. Evaluation of the neurotoxic effects of chronic embryonic exposure with inorganic mercury on motor and anxiety-like responses in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 2017, 59, 53–61.
- Lee, J.; Freeman, J.L. Embryonic exposure to 10 μg L−1 lead results in female-specific expression changes in genes associated with nervous system development and function and Alzheimer’s disease in aged adult zebrafish brain. Metallomics 2016, 8, 589–596.
- Zhu, B.; Wang, Q.; Shi, X.; Guo, Y.; Xu, T.; Zhou, B. Effect of combined exposure to lead and decabromodiphenyl ether on neurodevelopment of zebrafish larvae. Chemosphere 2016, 144, 1646–1654.
- Bault, Z.A.; Peterson, S.M.; Freeman, J.L. Directional and color preference in adult zebrafish: Implications in behavioral and learning assays in neurotoxicology studies. J. Appl. Toxicol. 2015, 35, 1502–1510.
- Xu, X.; Weber, D.; Burge, R.; Vanamberg, K. Neurobehavioral impairments produced by developmental lead exposure persisted for generations in zebrafish (Danio rerio). Neurotoxicology 2016, 52, 176–185.
- Tu, H.; Fan, C.; Chen, X.; Liu, J.; Wang, B.; Huang, Z.; Zhang, Y.; Meng, X.; Zou, F. Effects of cadmium, manganese, and lead on locomotor activity and neurexin 2a expression in zebrafish. Environ. Toxicol. Chem. 2017, 36, 2147–2154.
- Jiang, W.D.; Liu, Y.; Hu, K.; Jiang, J.; Li, S.H.; Feng, L.; Zhou, X.Q. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: Protective effects of myo-inositol. Aquat. Toxicol. 2014, 155, 301–313.
- Kirici, M.; Nedzvetsky, V.S.; Agca, C.A.; Gasso, V.Y. Sublethal doses of copper sulphate initiate deregulation of glial cytoskeleton, NF-kB and PARP expression in Capoeta umbla brain tissue. Regul. Mech. Biosyst. 2019, 10, 103–110.
- Pilehvar, A.; Town, R.M.; Blust, R. The effect of copper on behaviour, memory, and associative learning ability of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2020, 188, 109900.
- Ezeonyejiaku, C.D.; Obiakor, M.O.; Ezenwelu, C.O. Toxicity Of Copper Sulphate And Behavioral Locomotor Response Of Tilapia (Oreochromis niloticus) And Catfish (Clarias gariepinus) Species. Online J. Anim. Feed. Res. 2011, 1, 130–134.
- Baldissarelli, L.A.; Capiotti, K.M.; Bogo, M.R.; Ghisleni, G.; Bonan, C.D. Arsenic alters behavioral parameters and brain ectonucleotidases activities in zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 566–572.
- Mondal, P.; Shaw, P.; Dey Bhowmik, A.; Bandyopadhyay, A.; Sudarshan, M.; Chakraborty, A.; Chattopadhyay, A. Combined effect of arsenic and fluoride at environmentally relevant concentrations in zebrafish (Danio rerio) brain: Alterations in stress marker and apoptotic gene expression. Chemosphere 2021, 269, 128678.
- Dipp, V.R.; Valles, S.; Ortiz-Kerbertt, H.; Suarez, J.V.; Bardullas, U. Neurobehavioral Alterations in Zebrafish Due to Long-Term Exposure to Low Doses of Inorganic Arsenic. Zebrafish 2018, 15, 575–585.
- Sahu, G.; Kumar, V. The Toxic Effect of Fluoride and Arsenic on Behaviour and Morphology of Catfish (Clarias batrachus). Nat. Environ. Pollut. Technol. 2021, 20, 371–375.
- Nunes, B.; Capela, R.C.; Sérgio, T.; Caldeira, C.; Gonçalves, F.; Correia, A.T. Effects of chronic exposure to lead, copper, zinc, and cadmium on biomarkers of the European eel, Anguilla anguilla. Environ. Sci. Pollut. Res. 2014, 21, 5689–5700.
- Gioda, C.R.; Loro, V.L.; Pretto, A.; Salbego, J.; Dressler, V.; Flores, É.M.M. Sublethal zinc and copper exposure affect acetylcholinesterase activity and accumulation in different tissues of leporinus obtusidens. Bull. Environ. Contam. Toxicol. 2013, 90, 12–16.
- Yuan, Z.; Li, R.; Li, S.; Qiu, D.; Li, G.; Wang, C.; Ni, J.; Sun, Y.; Hu, H. Oxidative stress, neurotoxicity, and intestinal microbial regulation after a chronic zinc exposure: An experimental study on adult zebrafish (Danio rerio). Water Reuse 2023, 13, 82–96.
- Khan, A.T.; Weis, J.S. Effects of methylmercury on sperm and egg viability of two populations of killifish (Fundulus heteroclitus). Arch. Environ. Contam. Toxicol. 1987, 16, 499–505.
- Zubair, M.; Ahmad, M.; Saleemi, M.K.; Gul, S.T.; Ahmad, M.; Martyniuk, C.J.; Ullah, Q.; Umar, S. Sodium arsenite toxicity on hematology indices and reproductive parameters in Teddy goat bucks and their amelioration with vitamin C. Environ. Sci. Pollut. Res. 2020, 27, 15223–15232.
- Słomińska, I.; Jezierska, B. The effect of heavy metals on postembryonic development of common carp Cyprinus carpio L. Arch. Ryb. Pol. 2000, 8, 119–128.
- Witeska, M.; Jezierska, B.; Chaber, J. The influence of cadmium on common carp embryos and larvae. Aquaculture 1995, 129, 129–132.
- Witeska, M.; Sarnowski, P.; Ługowska, K.; Kowal, E. The effects of cadmium and copper on embryonic and larval development of ide Leuciscus idus L. Fish Physiol. Biochem. 2014, 40, 151–163.
- Jezierska, B.; Lugowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625–640.
- Sionkowski, J.; Łuszczek-Trojnar, E.; Popek, W.; Drąg-Kozak, E.; Socha, M. Impact of long-term dietary exposure to lead on some reproductive parameters of a female Common carp (Cyprinus carpio L.). Aquac. Res. 2017, 48, 111–122.
- Hontela, A.; Daniel, C.; Ricard, A.C. Effects of acute and subacute exposures to cadmium on the interrenal and thyroid function in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 1996, 35, 171–182.
- Chaurasia, S.S.; Gupta, P.; Kar, A.; Maiti, P.K. Lead induced thyroid dysfunction and lipid peroxidation in the fish Clarias batrachus with special reference to hepatic type I-5′-monodeiodinase activity. Bull. Environ. Contam. Toxicol. 1996, 56, 649–654.
- Bagdonas, E.; Vosylienė, M. A study of toxicity and genotoxicity of copper, zinc and their mixture to rainbow trout (Oncorhynchus mykiss). Biologija 2006, 1, 8–13.
- Cavas, T. In vivo genotoxicity of mercury chloride and lead acetate: Micronucleus test on acridine orange stained fish cells. Food Chem. Toxicol. 2008, 46, 352–358.
- Cavas, T.; Garanko, N.N.; Arkhipchuk, V.V. Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food Chem. Toxicol. 2005, 43, 569–574.
- Gautam, G.J.; Chaube, R.J. Differential effects of heavy metals (Cadmium, Cobalt, Lead and Mercury) on oocyte maturation and ovulation of the catfish Heteropneustes fossilis: An In Vitro Study. Turk. J. Fish. Aquat. Sci. 2018, 18, 1205–1214.
- Yan, W.; Hamid, N.; Deng, S.; Jia, P.P.; Pei, D.S. Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 397, 122795.
- Sierra-Marquez, L.; Espinosa-Araujo, J.; Atencio-Garcia, V.; Olivero-Verbel, J. Effects of cadmium exposure on sperm and larvae of the neotropical fish Prochilodus magdalenae. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2019, 225, 108577.
- Hayati, A.; Wulansari, E.; Armando, D.S.; Sofiyanti, A.; Amin, M.H.F.A.; Pramudya, M. Effects of in vitro exposure of mercury on sperm quality and fertility of tropical fish Cyprinus carpio L. Egypt. J. Aquat. Res. 2019, 45, 189–195.
- Dietrich, G.J.; Dietrich, M.; Kowalski, R.; Dobosz, S.; Karol, H.; Demianowicz, W.; Glogowski, J. Exposure of rainbow trout milt to mercury and cadmium alters sperm motility parameters and reproductive success. Aquat. Toxicol. 2010, 97, 277–284.
- Xie, D.; Chen, Q.; Gong, S.; An, J.; Li, Y.; Lian, X.; Liu, Z.; Shen, Y.; Giesy, J.P. Exposure of zebrafish to environmentally relevant concentrations of mercury during early life stages impairs subsequent reproduction in adults but can be recovered in offspring. Aquat. Toxicol. 2020, 229, 105655.
- Zhang, Q.F.; Li, Y.W.; Liu, Z.H.; Chen, Q.L. Exposure to mercuric chloride induces developmental damage, oxidative stress and immunotoxicity in zebrafish embryos-larvae. Aquat. Toxicol. 2016, 181, 76–85.
- Ibrahim, A.T.A.; Banaee, M.; Sureda, A. Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comparative biochemistry and physiology. Toxicol. Pharmacol. 2019, 225, 108583.
- Alkahemal-Balawi, H.F.; Ahmad, Z.; Al-Akel, A.S.; Al-Misned, F.; Suliman, E.-a.M.; Al-Ghanim, K.A. Toxicity bioassay of lead acetate and effects of its sub-lethal exposure on growth, haematological parameters and reproduction in Clarias gariepinus. Afr. J. Biotechnol. 2011, 10, 11039.
- Cao, J.; Wang, G.; Wang, T.; Chen, J.; Wenjing, G.; Wu, P.; He, X.; Xie, L. Copper caused reproductive endocrine disruption in zebrafish (Danio rerio). Aquat. Toxicol. 2019, 211, 124–136.
- Driessnack, M.K.; Jamwal, A.; Niyogi, S. Effects of chronic exposure to waterborne copper and nickel in binary mixture on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Chemosphere 2017, 185, 964–974.
- Adam, N.; Vakurov, A.; Knapen, D.; Blust, R. The chronic toxicity of CuO nanoparticles and copper salt to Daphnia magna. J. Hazard. Mater. 2015, 283, 416–422.
- Forouhar Vajargah, M.; Mohamadi Yalsuyi, A.; Sattari, M.; Prokic, M.; Faggio, C. Effects of Copper Oxide Nanoparticles (CuO-NPs) on Parturition Time, Survival Rate and Reproductive Success of Guppy Fish, Poecilia reticulata. J. Clust. Sci. 2020, 31, 499–506.
- Moosavi, M.J.; Shamushaki, V.-A.J. Effects of different levels of copper sulfate on growth and reproductive performances in guppy (P. reticulate). J. Aquac. Res. Dev. 2015, 6, 305.
- Shi, L.; Hu, X.; Wang, N.; Liang, H.; Wu, C.; Cao, H. Histopathological examination and transcriptome analyses to assess the acute toxic effects of arsenite exposure on rare minnows (Gobiocypris rarus). Ecotoxicology 2020, 29, 613–624.
- Nagato, E.G.; D’eon, J.C.; Lankadurai, B.P.; Poirier, D.G.; Reiner, E.J.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomics investigation of Daphnia magna responses to sub-lethal exposure to arsenic, copper and lithium. Chemosphere 2013, 93, 331–337.
- Smith, R.J.; Kollus, K.M.; Propper, C.R. Environmentally relevant arsenic exposure affects morphological and molecular endpoints associated with reproduction in the Western mosquitofish, Gambusia affinis. Sci. Total Environ. 2022, 830, 154448.
- Gárriz, Á.; Miranda, L.A. Effects of metals on sperm quality, fertilization and hatching rates, and embryo and larval survival of pejerrey fish (Odontesthes bonariensis). Ecotoxicology 2020, 29, 1072–1082.
- Gouva, E.; Nathanailides, C.; Skoufos, I.; Paschos, I.; Athanassopoulou, F.; Pappas, I.S. Comparative study of the effects of heavy metals on embryonic development of zebrafish. Aquac. Res. 2020, 51, 3255–3267.
- Gupta, G.; Srivastava, P.P.; Kumar, M.; Varghese, T.; Chanu, T.I.; Gupta, S.; Ande, M.P.; Jana, P. The modulation effects of dietary zinc on reproductive performance and gonadotropins’(FSH and LH) expression in threatened Asian catfish, Clarias magur (Hamilton, 1822) broodfish. Aquac. Res. 2021, 52, 2254–2265.
- Szarek-Gwiazda, E. Heavy metals contents in stone loach Noemacheilus barbatulus (L.) (Cobitidae) living in the river above and below dam reservoir (Dobczyce reservoir, southern Poland). Pol. J. Ecol. 1999, 47, 145–152.
- Ellenberger, S.A.; Baumann, P.C.; May, T.W. Evaluation of effects caused by high copper concentrations in Torch Lake, Michigan, on reproduction of yellow perch. J. Great Lakes Res. 1994, 20, 531–536.
- Miller, P.; Munkittrick, K.; Dixon, D. Relationship between concentrations of copper and zinc in water, sediment, benthic invertebrates, and tissues of white sucker (Catostomus commersoni) at metal-contaminated sites. Can. J. Fish. Aquat. Sci. 1992, 49, 978–984.
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Zhu, H.; Wang, Y. Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites 2022, 12, 60.
- Pelgrom, S.; Lamers, L.; Lock, R.; Balm, P.; Bonga, S.W. Interactions between copper and cadmium modify metal organ distribution in mature tilapia, Oreochromis mossambicus. Environ. Pollut. 1995, 90, 415–423.
- Allen, P. Accumulation profiles of lead and cadmium in the edible tissues of Oreochromis aureus during acute exposure. J. Fish Biol. 1995, 47, 559–568.
- Benoit, D.A.; Holcombe, G. Toxic effects of zinc on fathead minnows Pimephales promelas in soft water. J. Fish Biol. 1978, 13, 701–708.
- Devlin, E.W. Acute toxicity, uptake and histopathology of aqueous methyl mercury to fathead minnow embryos. Ecotoxicology 2006, 15, 97–110.
- Stouthart, A.J.H.X.; Spanings, F.a.T.; Lock, R.a.C.; Bonga, S.E.W. Effects of water pH on chromium toxicity to early life stages of the common carp (Cyprinus carpio). Aquat. Toxicol. 1995, 32, 31–42.
- Stouthart, A.; Spanings, F.; Lock, R.; Bonga, S.W. Effects of low water pH on lead toxicity to early life stages of the common carp (Cyprinus carpio). Aquat. Toxicol. 1994, 30, 137–151.
- Benoit, D.A. Toxic effects of hexavalent chromium on brook trout (Salvelinus fontinalis) and rainbow trout (Salmo gairdneri). Water Res. 1976, 10, 497–500.
- Beattie, J.; Pascoe, D. Cadmium uptake by rainbow trout, Salmo gairdneri eggs and alevins. J. Fish Biol. 1978, 13, 631–637.
- Michibata, H. Uptake and distribution of cadmium in the egg of the teleost, Oryzias latipes. J. Fish Biol. 1981, 19, 691–696.
- Sallam, M.; Zubair, M.; Tehseen Gul, S.; Ullah, Q.; Idrees, M. Evaluating the protective effects of vitamin E and selenium on hematology and liver, lung and uterus histopathology of rabbits with cypermethrin toxicity. Toxin Rev. 2020, 39, 236–241.
- Samson, J.C.; Shenker, J. The teratogenic effects of methylmercury on early development of the zebrafish, Danio rerio. Aquat. Toxicol. 2000, 48, 343–354.
- Slominski, A.; Ermak, G.; Mazurkiewicz, J.E.; Baker, J.; Wortsman, J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J. Clin. Endocrinol. Metab. 1998, 83, 1020–1024.
- Barjhoux, I.; Baudrimont, M.; Morin, B.; Landi, L.; Cachot, J. Effects of copper and cadmium spiked-sediments on embryonic development of Japanese medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 2012, 79, 272–282.
- El-Greisy, Z.A.; El-Gamal, A.H.A. Experimental studies on the effect of cadmium chloride, zinc acetate, their mixture and the mitigation with vitamin C supplementation on hatchability, size and quality of newly hatched larvae of common carp, Cyprinus carpio. Egypt. J. Aquat. Res. 2015, 41, 219–226.
- Sonnack, L.; Kampe, S.; Muth-Köhne, E.; Erdinger, L.; Henny, N.; Hollert, H.; Schäfers, C.; Fenske, M. Effects of metal exposure on motor neuron development, neuromasts and the escape response of zebrafish embryos. Neurotoxicol. Teratol. 2015, 50, 33–42.
- Monaco, A.; Capriello, T.; Grimaldi, M.C.; Schiano, V.; Ferrandino, I. Neurodegeneration in zebrafish embryos and adults after cadmium exposure. Eur. J. Histochem. 2017, 61, 2833.
- Wold, M.; Beckmann, M.; Poitra, S.; Espinoza, A.; Longie, R.; Mersereau, E.; Darland, D.C.; Darland, T. The longitudinal effects of early developmental cadmium exposure on conditioned place preference and cardiovascular physiology in zebrafish. Aquat. Toxicol. 2017, 191, 73–84.
- Ługowska, K.; Kondera, E. Developmental anomalies in ide (Leuciscus idus L.) larvae caused by copper and cadmium. Rocz. Nauk. Pol. Tow. Zootech. 2020, 16, 37–51.
- Sun, Y.; Li, Y.; Liu, Z.; Chen, Q. Environmentally relevant concentrations of mercury exposure alter thyroid hormone levels and gene expression in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Fish Physiol. Biochem. 2018, 44, 1175–1183.
- Cano-Viveros, S.; Galar-Martínez, M.; Gasca-Pérez, E.; García-Medina, S.; Ruiz-Lara, K.; Gómez-Oliván, L.M.; Islas-Flores, H. The relationship between embryotoxicity and oxidative stress produced by aluminum, iron, mercury, and their mixture on Cyprinus carpio. Water Air Soil Pollut. 2021, 232, 376.
- Wang, Y.; Shen, C.; Wang, C.; Zhou, Y.; Gao, D.; Zuo, Z. Maternal and embryonic exposure to the water soluble fraction of crude oil or lead induces behavioral abnormalities in zebrafish (Danio rerio), and the mechanisms involved. Chemosphere 2018, 191, 7–16.
- Curcio, V.; Macirella, R.; Sesti, S.; Ahmed, A.I.M.; Talarico, F.; Tagarelli, A.; Mezzasalma, M.; Brunelli, E. Morphological and Functional Alterations Induced by Two Ecologically Relevant Concentrations of Lead on Danio rerio Gills. Int. J. Mol. Sci. 2022, 23, 9165.
- Wirbisky, S.E.; Weber, G.J.; Lee, J.W.; Cannon, J.R.; Freeman, J.L. Novel dose-dependent alterations in excitatory GABA during embryonic development associated with lead (Pb) neurotoxicity. Toxicol. Lett. 2014, 229, 1–8.
- Li, X.; Chen, C.; He, M.; Yu, L.; Liu, R.; Ma, C.; Zhang, Y.; Jia, J.; Li, B.; Li, L. Lead Exposure Causes Spinal Curvature during Embryonic Development in Zebrafish. Int. J. Mol. Sci. 2022, 23, 9571.
- Shekari, S.; Sadooghi, M.; Hosseinzadeh, H. Effect Of Lead Chloride on Embryonic Stages and Kidney Differentiation in Pterophyllum Scalare. JAPAD 2014, 6, 53–62.
- Lasiene, K.; Straukas, D.; Vitkus, A.; Juodziukyniene, N. The influence of copper sulphate pentahydrate (CuSO4 5H2O) on the embryo development in the guppies (Poecilia reticulata). Ital. J. Anim. Sci. 2016, 15, 529–535.
- Kong, X.; Jiang, H.; Wang, S.; Wu, X.; Fei, W.; Li, L.; Nie, G.; Li, X. Effects of copper exposure on the hatching status and antioxidant defense at different developmental stages of embryos and larvae of goldfish Carassius auratus. Chemosphere 2013, 92, 1458–1464.
- Kabir, T.; Anwar, S.; Taslem Mourosi, J.; Hossain, J.; Rabbane, M.G.; Rahman, M.M.; Tahsin, T.; Hasan, M.N.; Shill, M.C.; Hosen, M.J. Arsenic hampered embryonic development: An in vivo study using local Bangladeshi Danio rerio model. Toxicol. Rep. 2020, 7, 155–161.
- Beaver, L.M.; Truong, L.; Barton, C.L.; Chase, T.T.; Gonnerman, G.D.; Wong, C.P.; Tanguay, R.L.; Ho, E. Combinatorial effects of zinc deficiency and arsenic exposure on zebrafish (Danio rerio) development. PLoS ONE 2017, 12, e01838312017.
- Lakshmanan, Y. Developmental Toxicity of Arsenic and its Underlying Mechanisms in the early Embryonic Development. Res. J. Pharm. Technol. 2016, 9, 340–344.
- Babich, R.; Van Beneden, R.J. Effect of arsenic exposure on early eye development in zebrafish (Danio rerio). J. Appl. Toxicol. 2019, 39, 824–831.
- Huang, W.; Cao, L.; Shan, X.; Xiao, Z.; Wang, Q.; Dou, S. Toxic Effects of Zinc on the Development, Growth, and Survival of Red Sea Bream Pagrus major Embryos and Larvae. Arch. Environ. Contam. Toxicol. 2010, 58, 140–150.
- Williams, N.D.; Holdway, D.A. The effects of pulse-exposed cadmium and zinc on embryo hatchability, larval development, and survival of Australian crimson spotted rainbow fish (Melanotaenia fluviatilis). Environ. Toxicol. 2000, 15, 165–173.
This entry is offline, you can click here to edit this entry!
