Intraprocedural stroke is a well-documented and feared potential risk of cardiovascular transcatheter procedures (TPs). Moreover, subclinical neurological events or covert central nervous system infarctions are concerns related to the development of dementia, future stroke, cognitive decline, and increased risk of mortality. Cerebral protection devices (CPDs) were developed to mitigate the risk of cardioembolic embolism during TPs. They are mechanical barriers designed to cover the ostium of the supra-aortic branches in the aortic arch, but newer devices are able to protect the descending aorta. CPDs have been mainly designed and tested to provide cerebral protection during transcatheter aortic valve replacement (TAVR), but their use in both Catheterization and Electrophysiology laboratories is rapidly increasing.
- stroke
- transcatheter procedures
- cerebral protection devices
- filter
- deflector
- supra-aortic
1. Introduction
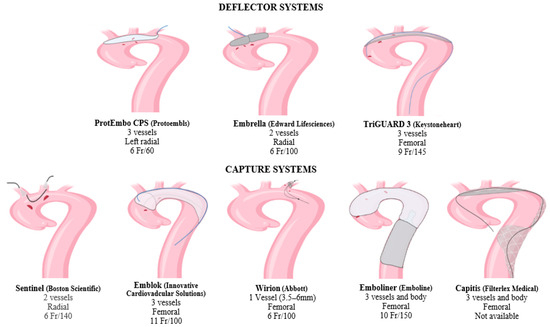
2. Deflector Systems
- –
-
Embrella (Edwards Lifesciences, Irvine, CA, USA) received a European CE mark approval in 2010. It was developed to deflect embolic material during TAVR [29]. This device is inserted by right radial or brachial approach with a 6 Fr sheath. The distal end is an umbrella-like device with two heparin-coated polyurethane membranes (pore size: 100 μm). The CPD is placed through the greater curvature of the aorta, safeguarding the brachiocephalic and left common carotid artery. Since the left subclavian artery is not covered by the device, Embrella provides only partial protection to supra-aortic vessels. According to the pilot study PROTAVI-C, the device was successfully positioned in 100% of the TAVR procedures (N = 41) [30]. Although its use was associated with a reduction in lesion volume evaluated by DW-MRI, it did not prevent the occurrence of new cerebral microemboli.
- –
-
TriGuard (Keystone Heart, Caesarea, Israel) received a European CE mark in 2014 [31]. It is advanced through a 9 Fr arterial sheath placed into the left femoral artery and deployed to cover the ostia of the three supra-aortic trunks. Its new generation, the TriGuard 3, incorporates a self-expanding deflection filter composed of a structural radiopaque nitinol frame and an ultra-thin polymer mesh (nominal pore size 115 × 145 μm). The device is heparin-coated to reduce thrombogenicity and increase lubricity. The full system includes a delivery subsystem for crimping and loading the device into an 8F sheath [32]. The device was primarily developed to provide cerebral protection during TAVR [33][34]. In recent years, its use in LAAC and VT ablation procedures has rapidly increased and provided encouraging results that could pave the way for new employment in electrophysiological procedures [35][36].
- –
-
ProtEmbo CPS (Protembis, Aachen, Germany, EU) received a European CE mark in 2014. This device covers all three supra-aortic vessels, and its low-profile design provides delivery by left radial access. The heparin-coated mesh has the smallest pore size (60 μm) among all available CPDs. For this reason, it might even safeguard the cerebrum from smaller-sized debris [32][37]. The PROTEMBO C trial evaluated the safety and performance of the ProtEmbo CPS in TAVR patients [38]. The CPD met the primary safety and performance endpoints compared to prespecified historical performance goals. Enrolled patients had smaller brain lesion volumes on DW-MRI compared to prior series and no large single lesions (>150 mm3). The ongoing PROTEMBO SF (ClinicalTrials.gov Identifier: NCT03325283) is a prospective, observational, multicenter, intention-to-treat study of the safety and feasibility of the ProtEmbo CPS in subjects with severe symptomatic native aortic valve stenosis indicated for TAVR.
3. Filter Systems
3.1. Supra-Aortic Filters
- –
-
Sentinel (Boston Scientific, Marlborough, MA, USA) received a European CE mark in 2014 and is the most widely used CPD so far. It is formed by a dual system filter basket containing two polyurethane mesh filters with 140 μm pores. It is advanced through a 6 Fr delivery catheter from the right radial over a 0.014 inch guidewire. It consists of a proximal filter (diameter of 9–15 mm) delivered in the brachiocephalic artery and a distal filter (diameter of 6.5–10 mm) delivered in the left common carotid artery. Through an articulating sheath, the device can be sealed into the aortic arch according to its anatomy [27]. Since the Sentinel device is deployed into supra-aortic vessels, the diameter of the supra-aortic vessels must be previously measured by CTA, because proximal and distal filters are developed to be accommodated within a brachiocephalic artery of 9 to 15 mm, and a common carotid of more than 3 mm [39]. The left vertebral artery remains unprotected. Sentinel devices have only one available size, so complete sealing might not be obtained in all aortic anatomies. Several uses of this device for LAAC and VT ablation have been reported [18][36].
- –
-
The Wirion (Abbott, Chicago, IL, USA) is a single filter usually employed for carotid stenting and lower extremity endovascular interventions [40]. It consists of a distal filter (filter basket and locking mechanism) and a rapid exchange delivery catheter. The exchange catheter has a 1.1 mm crossing profile and can be mounted on any 0.014 inch guidewire and via 6F or greater guiding catheters. The filter basket is made of a self-expanding nitinol scaffold and a nylon filter membrane with 100 μm pores. The filter can efficiently be deployed in vessels with a diameter ranging from 3.5 to 6.0 mm and at any location along the guidewire, using a proprietary remote locking system (handle at the proximal end of the delivery catheter). Since this device protects only one vessel at a time, it cannot be used alone for TPs at high risk of cardioembolism. A study reported the utility of Wirion in combination with Sentinel to complete the protection of the left vertebral artery in patients undergoing TAVR [31].
- –
-
Emblok Embolic Protection System (EPS, Innovative Cardiovascular Solutions, Grand Rapids, MI, USA) is currently only for investigational use. It is formed by an 11 F sheath device containing a 4 Fr pigtail catheter advanced through femoral access. The filter system is a 125 μm pore-size nitinol that allows the embolic filter and a radiopaque pigtail catheter to be advanced simultaneously through femoral access. It fits in various anatomies of the aorta with a diameter of up to 35 mm. The prospective, nonrandomized, multicenter, first-in-man pilot study was designed to evaluate the efficacy and safety of cerebral embolic protection utilizing the EPS-enrolled 20 patients undergoing TAVR [41]. The device was successfully placed and retrieved in all cases, and no neurological events were observed. Cerebral total new lesion volume was similar to other trials on cerebral protection during TAVR. An ongoing prospective, multicenter, single-blind, randomized controlled trial enrolling >500 patients aims to evaluate the safety, effectiveness, and performance of the EMBLOK EPS during TAVR by randomized comparison with a commercially available embolic protection device (ClinicalTrials.gov Identifier: NCT05295628).
3.2. Full Body Filters
- –
-
Emboliner (Emboline, Santa Cruz, CA, USA) device system is currently only for investigational use. It is advanced from a 9 Fr transfemoral sheath used for the 6 Fr pigtail catheter for TAVR. It is engineered to protect all three cerebral vessels and the whole body. Early results from the SafePass 2 trial were presented in Transcatheter Cardiovascular Therapeutics 2019, reflecting no adverse events at 30 days with 100% procedural success.
- –
-
Captis (Filterlex Medical, Caesarea, Israel) is currently under development and carries a deflector mechanism with ipsilateral transfemoral access. Positioned in the aortic arch and descending aorta, it promises to provide full cerebral and body protection. The results of the prospective, single-arm, first-in-human study presented at EuroPCR 2022 involving 20 patients who underwent TAVR showed 100% technical device performance success, including deploy and retrieve and any interferences with the TAVR procedure. There were neither device-related complications nor cerebrovascular events (ClinicalTrials.gov Identifier: NCT04659538).
| Device | Pros | Cons |
|---|---|---|
| ProtEmbo CPS [38] | Small size sheath (6 Fr); Left radial/brachial access; Mesh with the smallest pore size available; 100% successful device positioning. |
Partial coverage of the supra-aortic trunk; New cerebral lesions were detected, but smaller; Available evidence only for TAVR. |
| Embrella [30] | Small size sheath (6 Fr); Right radial/brachial access; 100% successful device positioning. |
Partial coverage of the supra-aortic trunk; New cerebral lesions were detected, but smaller; Available evidence only for TAVR. |
| TriGuard 3 [36][42] | Intermedium size sheath (9 Fr); Implantable through both the left and right femoral arteries; Full coverage of the supra-aortic trunk; Can be left in the aortic arch for days; 100% successful device positioning; Large amount of evidence; Available evidence for TAVR, LAAC, and VT ablation. |
Femoral access; Procedural concerns if transcatheter procedure performed through the retro-aortic path; New cerebral lesions were detected, but smaller. |
| Sentinel [36][43] | Small size sheath (6 Fr); Right radial/brachial access; 94.4% successful device positioning; Largest amount of evidence; Available evidence for TAVR, LAAC, and VT ablation. |
Partial coverage of the supra-aortic trunk; New cerebral lesions were detected but smaller. |
| Emblok [41] | Implantable through both the left and right femoral arteries; 100% successful device positioning. |
Intermedium size sheath (11 Fr); Femoral access; Procedural concerns if transcatheter procedure performed through the retro-aortic path; New cerebral lesions were detected, but smaller; Available evidence only for TAVR. |
| Wirion [31] | Small size sheath (6 Fr); Right radial/brachial access; Very low amount of evidence. |
Nonsufficient coverage of the supra-aortic trunk; Available evidence only for TAVR; |
| Emboliner | Coverage of the supra-aortic trunk and descending aorta; Implantable through both the left and right femoral arteries. |
Data on the first-in-man study is not yet available. |
| Capitis | Coverage of the supra-aortic trunk and descending aorta; Implantable through both the left and right femoral arteries. |
Data on the first-in-man study is not yet available. |
This entry is adapted from the peer-reviewed paper 10.3390/life13091819
References
- Davidson, L.J.; Davidson, C.J. Transcatheter Treatment of Valvular Heart Disease. JAMA 2021, 325, 2480–2494.
- Latib, A.; Mustehsan, M.H.; Abraham, W.T.; Jorde, U.P.; Bartunek, J. Transcatheter interventions for heart failure. EuroIntervention 2023, 18, 1135–1149.
- Canfield, J.; Totary-Jain, H. 40 Years of Percutaneous Coronary Intervention: History and Future Directions. J. Pers. Med. 2018, 8, 33.
- Wolpert, C.; Pitschner, H.; Borggrefe, M. Evolution of ablation techniques: From WPW to complex arrhythmias. Eur. Hear. J. Suppl. 2007, 9, I116–I121.
- Giustino, G.; Dangas, G.D. Stroke prevention in valvular heart disease: From the procedure to long-term management. EuroIntervention 2015, 14, W26–W31.
- Carrena, O.; Young, R.; Tarrar, I.H.; Nelson, A.J.; Woidyla, D.; Wang, T.Y.; Mehta, R.H. Trends in the Incidence and Fatality of Peripercutaneous Coronary Intervention Stroke. J. Am. Coll. Cardiol. 2022, 80, 1772–1774.
- Kogan, E.V.; Sciria, C.T.; Liu, C.F.; Wong, S.C.; Bergman, G.; Ip, J.E.; Thomas, G.; Markowitz, S.M.; Lerman, B.B.; Kim, L.K.; et al. Early Stroke and Mortality after Percutaneous Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation. Stroke 2023, 54, 947–954.
- Song, Z.-L.; Wu, S.-H.; Zhang, D.-L.; Jiang, W.-F.; Qin, M.; Liu, X. Clinical Safety and Efficacy of Ablation for Atrial Fibrillation Patients with a History of Stroke. Front. Cardiovasc. Med. 2021, 8, 630090.
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, Prevalence, Costs, and Impact on Disability of Common Conditions Requiring Rehabilitation in the United States: Stroke, Spinal Cord Injury, Traumatic Brain Injury, Multiple Sclerosis, Osteoarthritis, Rheumatoid Arthritis, Limb Loss, and Back Pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995.e1.
- Lansky, A.J.; Brown, D.; Pena, C.; Pietras, C.G.; Parise, H.; Ng, V.G.; Meller, S.; Abrams, K.J.; Cleman, M.; Margolis, P.; et al. Neurologic Complications of Unprotected Transcatheter Aortic Valve Implantation (from the Neuro-TAVI Trial). Am. J. Cardiol. 2016, 118, 1519–1526.
- Vermeer, S.E.; Prins, N.D.; Heijer, T.D.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Silent Brain Infarcts and the Risk of Dementia and Cognitive Decline. N. Engl. J. Med. 2003, 348, 1215–1222.
- Bokura, H.; Kobayashi, S.; Yamaguchi, S.; Iijima, K.; Nagai, A.; Toyoda, G.; Oguro, H.; Takahashi, K. Silent Brain Infarction and Subcortical White Matter Lesions Increase the Risk of Stroke and Mortality: A Prospective Cohort Study. J. Stroke Cerebrovasc. Dis. 2006, 15, 57–63.
- Maleki, K.; Mohammadi, R.; Hart, D.; Cotiga, D.; Farhat, N.; Steinberg, J.S. Intracardiac Ultrasound Detection of Thrombus on Transseptal Sheath: Incidence, Treatment, and Prevention. J. Cardiovasc. Electrophysiol. 2005, 16, 561–565.
- Mehta, R.I.; I Mehta, R.; E Solis, O.; Jahan, R.; Salamon, N.; Tobis, J.M.; Yong, W.H.; Vinters, H.V.; Fishbein, M.C. Hydrophilic polymer emboli: An under-recognized iatrogenic cause of ischemia and infarct. Mod. Pathol. 2010, 23, 921–930.
- Aldenhoff, Y.B.; Hanssen, J.H.; Knetsch, M.L.; Koole, L.H. Thrombus Formation at the Surface of Guide-Wire Models: Effects of Heparin-releasing or Heparin-exposing Surface Coatings. J. Vasc. Interv. Radiol. 2007, 18, 419–425.
- Frerker, C.; Schlüter, M.; Sanchez, O.D.; Reith, S.; Romero, M.E.; Ladich, E.; Schröder, J.; Schmidt, T.; Kreidel, F.; Joner, M.; et al. Cerebral Protection During MitraClip Implantation: Initial Experience at 2 Centers. JACC Cardiovasc. Interv. 2016, 9, 171–179.
- Feld, G.K.; Tiongson, J.; Oshodi, G. Particle formation and risk of embolization during transseptal catheterization: Comparison of standard transseptal needles and a new radiofrequency transseptal needle. J. Interv. Card. Electrophysiol. 2011, 30, 31–36.
- Heeger, C.; Metzner, A.; Schlüter, M.; Rillig, A.; Mathew, S.; Tilz, R.R.; Wohlmuth, P.; Romero, M.E.; Virmani, R.; Fink, T.; et al. Cerebral Protection during Catheter Ablation of Ventricular Tachycardia in Patients with Ischemic Heart Disease. J. Am. Heart Assoc. 2018, 7, e009005.
- Stachon, P.; Kaier, K.; Heidt, T.; Wolf, D.; Duerschmied, D.; Staudacher, D.; Zehender, M.; Bode, C.; Mühlen, C.v.Z. The Use and Outcomes of Cerebral Protection Devices for Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement in Clinical Practice. JACC Cardiovasc. Interv. 2021, 14, 161–168.
- Ahmad, Y.; Howard, J.P. Meta-Analysis of Usefulness of Cerebral Embolic Protection during Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 146, 69–73.
- Zahid, S.; Ullah, W.; Uddin, M.F.; Rai, D.; Abbas, S.; Khan, M.U.; Hussein, A.; Salama, A.; Bandyopadhyay, D.; Bhaibhav, B.; et al. Cerebral Embolic Protection during Transcatheter Aortic Valve Implantation: Updated Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101127.
- Kaur, A.; Dhaliwal, A.; Sohal, S.; Kliger, C.; Velagapudi, P.; Basman, C.; Dominguez, A.C. TCT-327 Clinical and Radiographic Measures of Stroke-Related Outcomes with Cerebral Embolic Protection Devices during TAVR: A Meta-Analysis. J. Am. Coll. Cardiol. 2022, 80, B131.
- Haussig, S.; Mangner, N.; Dwyer, M.G.; Lehmkuhl, L.; Lücke, C.; Woitek, F.; Holzhey, D.M.; Mohr, F.W.; Gutberlet, M.; Zivadinov, R.; et al. Effect of a Cerebral Protection Device on Brain Lesions following Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis: The CLEAN-TAVI Randomized Clinical Trial. AMA 2016, 316, 592–601.
- Sharma, S.P.; Cheng, J.; Turagam, M.K.; Gopinathannair, R.; Horton, R.; Lam, Y.-Y.; Tarantini, G.; D’Amico, G.; Rofastes, X.F.; Lange, M.; et al. Feasibility of Left Atrial Appendage Occlusion in Left Atrial Appendage Thrombus: A Systematic Review. JACC Clin. Electrophysiol. 2020, 6, 414–424.
- Agrawal, A.; Isogai, T.; Shekhar, S.; Kapadia, S. Cerebral Embolic Protection Devices: Current State of the Art. US Cardiol. Rev. 2023, 17, e02.
- Kang, G.; Lee, J.; Song, T.; Pantelic, M.; Reeser, N.; Keimig, T.; Nadig, J.; Villablanca, P.; Frisoli, T.; Eng, M.; et al. 3-Dimensional CT Planning for Cerebral Embolic Protection in Structural Interventions. JACC Cardiovasc. Imaging 2020, 13, 2673–2676.
- Cubero-Gallego, H.; Pascual, I.; Rozado, J.; Ayesta, A.; Hernandez-Vaquero, D.; Diaz, R.; Alperi, A.; Avanzas, P.; Moris, C. Cerebral protection devices for transcatheter aortic valve replacement. Ann. Transl. Med. 2019, 7, 584.
- Steinvil, A.; Benson, R.T.; Waksman, R.; Chhatriwalla, A.K.; Allen, K.B.; Saxon, J.T.; Cohen, D.J.; Aggarwal, S.; Hart, A.J.; Baron, S.J.; et al. Embolic Protection Devices in Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2016, 9, e003284.
- Demir, O.M.; Iannopollo, G.; Mangieri, A.; Ancona, M.B.; Regazzoli, D.; Mitomo, S.; Colombo, A.; Weisz, G.; Latib, A. The Role of Cerebral Embolic Protection Devices During Transcatheter Aortic Valve Replacement. Front. Cardiovasc. Med. 2018, 5, 150.
- Rodés-Cabau, J.; Kahlert, P.; Neumann, F.-J.; Schymik, G.; Webb, J.G.; Amarenco, P.; Brott, T.; Garami, Z.; Gerosa, G.; Lefèvre, T.; et al. Feasibility and Exploratory Efficacy Evaluation of the Embrella Embolic Deflector System for the Prevention of Cerebral Emboli in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2014, 7, 1146–1155.
- Nombela-Franco, L.; Armijo, G.; Tirado-Conte, G. Cerebral embolic protection devices during transcatheter aortic valve implantation: Clinical versus silent embolism. J. Thorac. Dis. 2018, 10, S3604–S3613.
- Gasior, T.; Mangner, N.; Bijoch, J.; Wojakowski, W. Cerebral embolic protection systems for transcatheter aortic valve replacement. J. Interv. Cardiol. 2018, 31, 891–898.
- Baumbach, A.; Mullen, M.; Brickman, A.M.; Aggarwal, S.K.; Pietras, C.G.; Forrest, J.K.; Hildick-Smith, D.; Meller, S.M.; Gambone, L.; den Heijer, P.; et al. Safety and performance of a novel embolic deflection device in patients undergoing transcatheter aortic valve replacement: Results from the DEFLECT I study. EuroIntervention 2015, 11, 75–84.
- Samim, M.; van der Worp, B.; Agostoni, P.; Hendrikse, J.; Budde, R.P.; Nijhoff, F.; Ramjankhan, F.; Doevendans, P.A.; Stella, P.R. TriGuard™ HDH embolic deflection device for cerebral protection during transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2017, 89, 470–477.
- Zachariah, D.; Limite, L.R.; Mazzone, P.; Marzi, A.; Radinovic, A.; Baratto, F.; Italia, L.; Ancona, F.; Paglino, G.; Della Bella, P. Use of Cerebral Protection Device in Patients Undergoing Ventricular Tachycardia Catheter Ablation. JACC Clin. Electrophysiol. 2022, 8, 528–530.
- Berg, J.; Preda, A.; Fierro, N.; Marzi, A.; Radinovic, A.; Della Bella, P.; Mazzone, P. A Referral Center Experience with Cerebral Protection Devices: Challenging Cardiac Thrombus in the EP Lab. J. Clin. Med. 2023, 12, 1549.
- Jagielak, D.; Targonski, R.; Ciecwierz, D. First-in-Human Use of the Next-generation ProtEmbo Cerebral Embolic Protection System During Transcatheter Aortic Valve-in-valve Implantation. Interv. Cardiol. Rev. Res. Resour. 2021, 16, 1–4.
- Jagielak, D.; Targonski, R.; Frerker, C.; Abdel-Wahab, M.; Wilde, J.; Werner, N.; Lauterbach, M.; Leick, J.; Grygier, M.; Misterski, M.; et al. Safety and performance of a novel cerebral embolic protection device for transcatheter aortic valve implantation: The PROTEMBO C Trial. EuroIntervention 2022, 18, 590–597.
- Vernikouskaya, I.; Rottbauer, W.; Gonska, B.; Rodewald, C.; Seeger, J.; Rasche, V.; Wöhrle, J. Image-guidance for transcatheter aortic valve implantation (TAVI) and cerebral embolic protection. Int. J. Cardiol. 2017, 249, 90–95.
- Giannopoulos, S.; Armstrong, E.J. WIRION™ embolic protection system for carotid artery stenting and lower extremity endovascular intervention. Futur. Cardiol. 2020, 16, 527–538.
- Latib, A.; Mangieri, A.; Vezzulli, P.; Spagnolo, P.; Sardanelli, F.; Fellegara, G.; Pagnesi, M.; Giannini, F.; Falini, A.; Gorla, R.; et al. First-in-Man Study Evaluating the Emblok Embolic Protection System During Transcatheter Aortic Valve Replacement. JACC: Cardiovasc. Interv. 2020, 13, 860–868.
- Nazif, T.M.; Moses, J.; Sharma, R.; Dhoble, A.; Rovin, J.; Brown, D.; Horwitz, P.; Makkar, R.; Stoler, R.; Forrest, J.; et al. Randomized Evaluation of TriGuard 3 Cerebral Embolic Protection After Transcatheter Aortic Valve Replacement: REFLECT II. JACC Cardiovasc. Interv. 2021, 14, 515–527.
- Kapadia, S.R.; Kodali, S.; Makkar, R.; Mehran, R.; Lazar, R.M.; Zivadinov, R.; Dwyer, M.G.; Jilaihawi, H.; Virmani, R.; Anwaruddin, S.; et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017, 69, 367–377.
