Asthma and adolescence are two sensitive points and are difficult to manage when they coexist. The first is a chronic respiratory condition, with frequent onset in early childhood (between 3 and 5 years), which can improve or worsen with age. Adolescence is the period between childhood and adulthood (12–19 years), marked by various internal and external conflicts and a limited capacity to understand and accept any aspect that is delimited by the pattern of the social circle (of the entourage) frequented by the individual. Therefore, the clinician is faced with multiple attempts regarding the management of asthma encountered during the adolescent period, starting from the individualization of the therapy to the control of compliance (which depends equally on the adverse reactions, quality of life offered and support of the close circle) and the social integration of the subject, communication probably having a more important role in the monitoring and evolution of the condition than the preference for a certain therapeutic scheme.
1. Introduction
Asthma includes respiratory symptoms caused by hyperreactivity to stimuli, such as wheezing, difficult breathing, expiratory dyspnea, a feeling of tightness in the chest and coughing, having an undulating evolution, marked by frequent relapses and remissions, dependent on environmental factors (physical exercises, smoke, pollen, mold, pollution, microbes, weather changes, strong emotions) [5,6]. It often occurs in association with other allergies (due to the common pathogenic mechanism mediated by IgE), such as allergic rhinitis, conjunctivitis, atopic dermatitis and food allergies, and also with non-allergic disorders, including obesity, gastroesophageal reflux and psychiatric disorders [5]. Respiratory symptoms unresponsive to therapy (to be distinguished from uncontrolled asthma, defined by poor symptom control and/or frequent exacerbations requiring hospitalization) should draw the clinician’s attention for reevaluation to rule out not only cystic fibrosis (CF), non-CF bronchiectasis, immunodeficiency, primary ciliary dyskinesia, bacterial bronchitis or bronchiolitis obliterans, foreign bodies and vocal cord dysfunction, but also psychological problems [7,8]. For children over 6 years and adolescents, four major clinical phenotypes have been described: early-onset allergic asthma, moderate-to-severe early-onset allergic asthma, late-onset nonallergic eosinophilic asthma and late-onset nonallergic noneosinophilic asthma [9].
2. Epidemiology
The clearest orientation of the prevalence of asthma in children around the world was outlined by the International Study of Asthma and Allergies in Childhood (ISAAC), which showed significant geographic variations. Thus, the IIIa phase of the study (carried out between 2000 and 2003) ranked the English-speaking countries and Latin American countries in the first places in terms of the diagnosis rate of asthma, the opposite poles being Africa, the Indian subcontinent and the eastern Mediterranean, regions where asthma is less frequently detected, but in more severe forms [
1]. The prevalence of chronic asthma in childhood increases inversely proportional to income (relative to the federal poverty threshold (FPL)); thus, it is 8.2% among families with incomes above 200% of the FPL, and 12.2% among those with incomes below 100% of the FPL. In terms of race/ethnicity differences, Black children have a higher overall incidence of asthma [
6]. Boys seem to be more prone than girls to exhibit early-onset asthma and pulmonary flow impairment, with the exception of FVC, regardless of ethnicity [
10].
In calculating the prevalence, the degrees of underdiagnosis and overdiagnosis must also be taken into account, the quality of life being directly proportionally influenced by these due to incorrectly administered treatment. Aaron et al. have outlined, based on their own studies and from the literature, the potential causes that lead to diagnostic errors, among which it can mention the underreporting of symptoms to the family doctor due to not only the poor perception of the airflow limit and the poverty of respiratory symptoms (31%), diagnostic sensitivity, poor spirometry (sensitivity of 29%, positive predictive value of 77% and negative predictive value of 53%) and low socioeconomic status, but also the impossibility of using objective pulmonary function tests at the time of diagnosis (which leads to an increase in false diagnoses of asthma between 48% and 54%), sustained remission (found in 12% of the study subjects, previously tested positive for asthma and mentioned in the literature as being between 68% and 25%, increasing with the early onset of symptoms) and obesity (rate of error in diagnosis being similar to that of the non-obese population, although other authors put it between 25% and 41%) [
11].
3. Pathogenesis
The National Asthma and Education and Prevention Program Expert Panel Report 3 defines asthma as “a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role: in particular, mast cells, eosinophils, neutrophils (especially in sudden onset, fatal ex-acerbations, occupational asthma, and patients who smoke), T lymphocytes, macrophages, and epithelial cells” [
13]. The cause of bronchial asthma is not fully known, but the risk factors (including genetic ones, viral respiratory infections in infants, atopy, changes in the microbiome, abnormalities of vitamin D metabolism) and gene–environment interactions (pollution, stress, exposure to chemicals) play important roles in the pathogenesis of asthma [
14,
15].
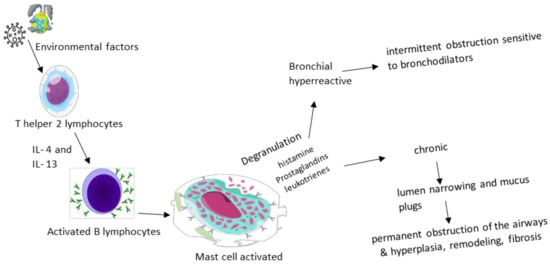
There are two phases of asthma exacerbation: early and late. The pathological process is initiated by IgE antibodies sensitized to certain triggering factors in the environment and released by plasma cells, which then bind to mast cells and basophils with high affinity, causing degranulation and the release of histamine, prostaglandins and leukotrienes, a mechanism that causes the intermittent obstruction of the flow of air. Th2 lymphocytes maintain the process by producing interleukins (IL-4, IL-5 and IL-13—markers of remodeling, fibrosis and hyperplasia) and GM-CSF, supporting inflammation. The late phase represents the concentration at the lung level not only of eosinophils, basophils, neutrophils and T helper and memory cells that maintain bronchoconstriction and inflammation, but also of mast cells that play a role in attracting late-phase reactants to inflamed sites [
6,
17]. Eosinophilic inflammation, an excess of mucus, and the remodeling of large airways determined a thickened reticular basement membrane and increased airway smooth muscle only in 1/3 of fatal asthma cases, and exclusively in large airways (in correlation with the duration of asthma) [
18].
Thus, Barrios RJ. et al., bring to the fore, through a centralization of studies from the last decades, two physiopathological mechanisms based on Th2 action, subdividing the obstructive process according to not only the type of hypersensitivity response (1 versus 4) and the interaction with the targeted receptors (immunoglobulin receptors, leukotrienes or the α chain of the IL-4 receptor), but also the involved interleukins (IL-4, IL-5, IL-9, IL-13). The role played by IL-4 is also emphasized in the production of immunoglobulin E (IgE) antibodies, the target of which is the immune cells that they sensitize for a subsequent encounter with the pathogen, at which point degranulation and the release of inflammatory molecules occur with both direct involvement (amplification of hypersensitivity, glycoprotein production and eosinophilia, similar to leukotrienes) and indirect involvement by stimulating mast cell maturation and IgE secretion [
19,
20]. Mast cell degranulation occurs as a result of the cross-link formed between two IgE molecules and the causative antigen, which, together with eosinophils activated by means of IL-5, maintain the inflammatory process by means of cytokines [
21]. At the opposite pole of the immune mechanism are regulatory T lymphocytes (Treg) that influence the expressions of CD 25, the transcription factor forkhead box P3, transforming growth factor beta (TGF-β) and interleukin 10 (IL-10), components that play a role in modulating the immune tolerance. Beyond these, the regulatory B cell population is also being studied for activity in human subjects with asthma [
22].
Despite the previous considerations, the current research contradicts the hypothesis of the sterility of the lower airways, an aspect that equally affects the entire range of lung pathologies delimited, especially asthma. With reference to it, Sullivan A. et al. discuss the disruption of bacterial diversity in the lungs of asthmatics, which is characterized by the intensification of the presence of microorganisms such as
Haemophilus,
Neisseria,
Moraxella and
Staphylococcus, doubled by the decrease in
Streptococcus species with
Veillonella,
Faecalibacterium,
Lachospira and
Rothia, disturbances that can still be registered from early childhood, as they are considered risk factors for the subsequent development of asthma or sensitivity to allergens. The circadian rhythm also seems to influence the respiratory microbiota, an aspect objectified by the study of the Bmal 1 gene at the level of the bronchiolar epithelium, which modulates the activity of neutrophils and the host’s response to pathogens, such as
Streptococcus pneumoniae [
23,
24,
25]. Collaborating with the data presented above,
Figure 1 highlights the physiopathological cascade underlying the acute and chronic symptoms found in bronchial asthma.
Figure 1. Physiopathological cascade in asthma.
4. Diagnosis
The diagnosis depends on the complexity of the case and the age of the patient, being accessible for the child with characteristic symptoms and cooperative and responding to therapy, and complex for the small child with recurrent wheezing. Asthma is mainly diagnosed clinically when symptoms can be reversed via the administration of a short-acting β2-agonist (SABA), in the absence of another disorder that can cause airflow obstruction.
Chest X-ray does not change the clinical approach and therapeutic decision in acute asthma crisis, being able to indicate, in the case of SpO
2 92% or fever, pathologies such as pneumonia, atelectasis or pneumothorax. Chest X-ray can help exclude some differential diagnoses, such as respiratory infections, foreign body inhalation or congenital diseases (cardiac, lobar emphysema) [
27]. Airway narrowing can also be assessed using CT imaging, single-photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI) and optical coherence tomography (OCT), a new technique that involves the examination of the airways at the time of bronchoscopy and provides insight into the structure of their walls and the mechanical properties with an impact on their narrowing [
28].
5. Treatment
For a correct orientation regarding therapy and monitoring, asthma can be subdivided into type 2 (high) and non-type 2 (low) endotypes based on their underlying inflammatory pathways [
9].
Medications used to control background symptoms and asthma exacerbations in adolescent patients include inhaled corticosteroids (ICSs), short-acting inhaled bronchodilators (SABAs), long-acting β2-adrenergic receptor agonists (LABAs), long-acting muscarinic receptor antagonists, leukotriene receptors (LTRAs) and, for more serious forms of the disease, specific monoclonal antibodies (IgE (Omalizumab), IL-5 (Mepolizumab, Reslizumab, Benralizumab) and IL-4/IL-13 (Dupilumab)) [
5,
9,
30]. Monoclonal antibodies have the clinical effect of increasing FEV
1 and quality of life, along with decreasing the need for inhaled corticosteroids and hospitalization. It should also be mentioned that Omalizumab can be administered after the age of 6 years, while Mepolizumab, Benralizumab and Dupilumab can be given after 12 years, and Reslizumab after 18 years [
31,
32].
Currently, the treatment of the adolescent patient with asthma comprises two stages: the initial treatment, instituted at the first evaluation of the patient, and the subsequent treatment guided by the evolution of the disease. The first treatment regimen is chosen according to the presence and frequency of day/night symptoms, the limitation of physical activity and the risk of exacerbations. Afterwards, it is based on the patient’s response to the administered treatment plan, asthma being classified as controlled, partially controlled or uncontrolled [
33]. The choice of their optimal way of administration is made depending on the clinical form of asthma.
Depending on the type of asthma, there are various biomarkers used to evaluate the adherence to therapy, essentially for adolescent monitoring. For type 2-high asthma, potential biomarkers could be serum allergen-specific IgE (SIgE), fractional exhaled nitric oxide (FeNO), the blood eosinophile count, sputum, bronchoalveolar lavage (BAL) or a bronchial biopsy, as well as some cytokines (IL-4, IL-5 and IL-13) or some cytokines specific to the innate immune system (IL-25, IL-33 and TSLP). In contrast, the diagnosis for type 2-low asthma is more challenging.
Comorbidities that affect asthma control can include rhinosinusitis, obesity, gastroesophageal reflux disease, obstructive apnea, psychological disorders as well as medications (angiotensin-converting receptor blockers—ACE-, B-blockers, aspirin and other NSAIDs) [
31].
Being one of the basic drugs both in the treatment of acute asthma exacerbations and in the long term, systemic or inhaled corticosteroids must be carefully evaluated in terms of the risk/benefit ratio, their over-administration being burdened by increased mortality and adverse effects, such as osteopenia, osteoporosis, muscle atrophy and dyspepsia, impacts on the cardiovascular function, growth curve or visual acuity, the increased incidence of diabetes, obesity, infections and psychological manifestations (depression, anxiety, sleep disorders) as well as the suppression of the adrenal function, manifestations that partially overlap with the aspects related to the non-pharmacological management of patients, developed below. The harmful effects seem to be directly correlated with the frequency of use in the therapeutic scheme, their cumulative nature being still under research [
39].
The physiopathological stage targeted by corticotherapy is represented by the production of pro-inflammatory mediators, the stimulation of chemotaxis, adhesion molecules and the antigen–receptor interaction, a stage that it mitigates through its anti-inflammatory role exercised through transrepression. At the molecular level, it notes the reduction in eosinophilia, the increase in mRNA degradation, the synthesis of anti-inflammatory proteins and the reduction in vascular permeability with the consequent reduction in liquid extravasation and the stopping of the remodeling process [
39,
41].
The effectiveness of corticosteroid therapy is influenced by the non-pharmacological conditions to which the patient is exposed, namely, the adherence to therapy and correct inhalation technique, continued exposure to allergenic stimuli in the environment (which can induce corticosteroid resistance mediated by IL-2 and IL-4), associated comorbidities as well as genetic variability (e.g., T-box 21 variants, Fc fragment of the IgE II receptor, histone deacetylase 1, dual-specificity phosphatase 1). Thus, a series of predictive factors of the response to corticotherapy have been outlined over time, which, in the pediatric population, are represented by the pulmonary function at the time of the initiation of therapy, inflammatory markers, the gene functionality or the degree of sensitization to allergens [
41].
Therapy based on systemic/inhaled corticosteroids has aroused the interest of researchers regarding the possibility of the similar use of magnesium in refractory bronchial asthma, and specifically moderate/severe bronchiolitis. However, no statistical results were observed to encourage this practice [
43,
44].
Therefore, another therapeutic class used in the control of pediatric asthma is represented by muscarinic receptor agonists (anticholinergic). These exert influence through their action on the M1/M2/M3 receptors that modulate the neuronal and non-neuronal signals of acetylcholine, the tone of the respiratory muscles (reducing bronchoconstriction), the mucous secretion of the glands as well as inflammation or remodeling. With regard to the method of administration, one can opt for monotherapy or combined therapy with β-agonists (e.g., Tiotropium/Olodaterol, Aclidinium/Formoterol, Glicopyronium/Indacaterol), the combined preparation form, as well as the long-acting one, recording promising results in the control of asthmatic exacerbations in children over 2 years of age and of long-term symptoms. It is also worth mentioning the superiority of monotherapy with β2-agonists, compared to anticholinergics, when opting for this [
52,
53,
54].
Having as the main representatives Montelukast, Zafirlukast, Pranlukast or Zileuton, the asthma medications that act at the level of leukotriene receptors with the aim of suppressing production and antagonizing inflammation are divided, depending on the therapeutic target, into G protein-coupled receptor antagonists or inhibitors of 5-lipoxygenase, with action at the level of leukotrienes B4, C4, D4 and E4 [
53]. Montelukast, a selective antagonist of the D4 receptor, has proven its effectiveness in bronchial asthma compared to placebos, its administration reducing the need for corticotherapy in controlled forms of asthma. Presenting a peak of action approximately two and a half hours post-administration and at a half-life of 3–4 h, the therapeutic dose in pediatrics is 4 mg/day (2–5 years)–5 mg/day (6–14 years), without the need for adjustment in cases of impaired renal or hepatic function (due to excretion mainly through the biliary tract, except in severe cases).
With relatively little therapeutic experience, partly due to the recent introduction in management protocols, biological agents target individual stages of the physio-pathological cascade, an aspect from which arises the need to study and know the phenotypic particularities of asthma in order to choose the appropriate preparation. The best-known biological agents are Omalizumab (which acts on IgE, reducing the immune response and, consequently, allergic symptoms), Dupilumab (which blocks the IL-4 and IL-13 pathways), Mepolizumab, Reslizumab and Benralizumab (which targets the IL-5 pathway, thus decreasing eosinophilic proliferation) [
53]. Antibiotics from the macrolide class (Azithromycin, Clarithromycin) can be added to their support, which, in addition to their anti-inflammatory and antimicrobial roles, have modulatory effects on adhesion molecules and the neutrophil function, and also have a prophylactic function in asthmatic exacerbations caused by respiratory infections with
Chlamydia or
Mycoplasma pneumoniae [
65].
Summarizing the above, the differences from a therapeutic and pharmacological point of view between different age groups reside mainly in the therapeutic classes proven to be effective and safe among them. Thus, in early childhood, the main therapeutic modality is aimed at steroids. Other therapeutic strategies include short-acting inhaled bronchodilators (SABAs), long-acting β2-adrenergic receptor agonists (LABAs) and long-acting muscarinic receptor antagonists and leukotriene receptors (LTRAs). With advancing age, the current guidelines recommend the introduction of monoclonal antibodies to the therapeutic scheme. This introduction is performed successively, having as essential age steps the ages of 6 years (Omalizumab), 12 years (Mepolizumab, Benralizumab, Dupilumab) and 18 years (Reslizumab). At the opposite pole, the treatment of adults aims at the same broad lines; the difference lies not only in a greater variety of monoclonal antibodies that can not only be addressed in therapy, but also in the need to adjust doses according to comorbidities and chronic therapy associated with advanced age. From a pharmacological point of view, although the means of action of the substances are varied, they do not have different administration implications depending on age. The primary difference is the adaptation of the dose and combinations according to body weight/comorbidities/severity, as well as the knowledge of and strict adherence to the parameters of efficiency and safety (especially with regard to modern monoclonal antibody therapies) dictated by the current studies, with statistical relevance.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11092429