Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
ACE2 coupling, and the S protein cleavage site, as targets for the development of nano-enabled solutions that, for example, prevent viral attachment and fusion with the host cell by either blocking viral RBD/spike proteins or cellular ACE2 receptors.
- SARS-CoV-2
- mutations
- variants
- clinical resurgence
- nanotechnology
1. Escape Mutations and Clinical Waves of COVID-19
Detailed investigations of different COVID-19 waves in their respective geographic locations have revealed close associations with mutations of virion at its spike glycoprotein. Such mutations have led to the emergence of several variants, such as B.1.1.7, B.1.351, P.1, B.1.617, B.1.1.529, BA.1, BA.2, BA.3, BA.4, BA.5, BA.2.75 (x), BQ.1, XBB (z), XBB.1.5-like, CH.1.1, XBB.1.16, and XBB.1.5-like + F456L [1][2][3][4]. Most of these variants appeared to be more infectious and virulent than the original Wuhan virus.
It has been reported that antibodies developed from natural infection with a variant or through vaccination are less effective at neutralizing other mutants [2][5][6][7]. The emergence of such variants has been attributed to several mutations in the SARS-CoV-2 spike protein, i.e., in the K417, L452, K477, T478, E484, Q498, and N501 region of the RBD [3][8][9]. On the basis of single-nucleotide polymorphisms (SNPs), alterations in amino acid residues in the same RBD region are considered a major cause of the mutation-dependent emergence of variants [9]. In the case of Alpha-N501Y, a residue N amino acid at position 501 was replaced with a Y, whereas Beta-K417N, -E484K, and -N501Y originated from mutations at residues 417, 484, and 501, respectively, where K, E, and N were replaced with N, K, and Y acid [10]. Likewise, Gamma-K417T, -E484K, and -N501Y exhibited mutations at residues 417, 484, and 501, where K, E, and N acids were replaced with T, K, and Y acids, respectively [7]. In the Delta-K417N, -L452R, -T478K, and -E484Q strains, mutations occurred at positions 417, 452, 478, and 484, with N, R, K, and Q replacing K, L, T, and E, respectively [3][9][11][12]. Similarly, in Omicron-K417N, -K477N, -T478K, -E484A, -Q498R, and -N501Y, K, T, E, Q, and N at residues 417, 477, 478, 484, 498, and 501 were replaced with N, K, A, R, and Y [3][13]. All mutants exhibit the ability to escape neutralizing antibodies, and the vaccines developed as of today, therefore, do not offer full immune protection [2]. More immune-escape mutations might emerge while the pandemic situation progresses [2], and while antibody responses to SARS-CoV-2 receptor-binding sites are strong enough to neutralize the original Wuhan strain, mutated variants can elude this response. Hence, it does not seem feasible to prevent the spread of SARS-CoV-2 through regular updates of the available vaccines in response to the emergence of new mutants, which is why the whole world is looking for alternative technological solutions to the ongoing pandemic.
2. Importance of Nanotechnology
Nanotechnology is the method for controlling molecules below 100 nm scale for enhancing desired functionality. In the material world, nanomaterial lies in the scale of ≥1 nm to ≤100 nm. In the 21st century, nanotechnology has been considered the most attractive tool in many fields, including engineering, biology, chemistry, and physics [14]. Nowadays, nanomaterial science, electronics and nanoscale engineering (ENE), nano-agriculture, nanomedicine, nano-biotechnology (NBT), nano-robotics, nano-machines, and nano-toxicology have been established as branches of nanotechnology [14][15][16][17][18][19][20]. Nanotechnology offers numerous advantageous features for many biomedical applications, like enhanced functionality through their increased volume aspect ratio and durability in action and targeted delivery through precise selectivity [21][22][23] (Figure 1).
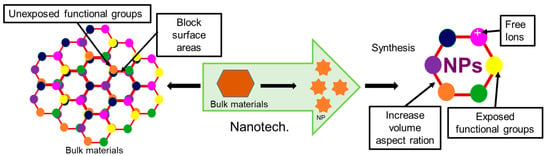
Figure 1. Advantages of the nanoparticle compared to their bulk material.
Recently, nanotechnology has emerged as one of the most promising technologies on account of its ability to deal with viral diseases in an effective manner, addressing the limitations of traditional antiviral medicines. It has not only enabled researchers to overcome problems related to the solubility and toxicity of drugs but also imparted unique properties to drugs, which in turn has increased their potency and selectivity toward viral particles against the host cells [24]. Overall, antivirals coated with nanoparticles offer several advantageous features compared to non-coated antivirals, like increased cellular uptake capability of drugs due to increased ion exchangeability of NPs, decreased doses of drugs due to precise selectivity of NPs through targeted delivery of drugs, increased cellular influx and decreased efflux, increased durability of action of Nanoparticle coated drugs through their slow release, and increased antiviral activity through targeted modification of functional groups [14][16][23].
In spite of its many advantages, this exciting technology still has many limitations, such as the unavailability of biocompatible, biodegradable, and eco-friendly nanomaterials [25][26][27], and most chemically synthesized metal and metal oxide nanomaterials being unsuitable for application in biological systems [26]. Their stability and durability of action is another challenge, because of their relatively short half-life [25]. The biocompatibility and toxicity of inorganic nanoparticles should thus be assessed before applications are implemented in living systems. Biocompatibility assays could be performed using in vitro cell culture systems or in vivo live animal models. In vitro virus neutralization tests are essential to confirm antiviral activity, and in vivo live animal models are needed to determine physical, biological, and histopathological changes as well as the efficacy, safety, half-life, and shelf-life of nano-drugs. Eco-friendly green synthesis protocols using naturally available materials could be an alternative to chemical synthesis processes. The desired shelf-life of a nanomedicine could be adjusted by controlling the size, shape, charge, and surface chemistry of the nanomaterials. Likewise, toxicity could be minimized by adjusting the particle size: for example, 1.4 nm-sized Au NPs and Ag NPs are toxic, while 15 nm-sized NPs are nontoxic for living systems [28]. Lack of knowledge and awareness about the use of nanomedicine is another limitation of this promising technology.
3. Antiviral Nanomaterials That Prevent Viral Infections
3.1. Inhibition of Receptor-Mediated Host–virus Attachments and Cell Fusion
In the case of the SARS-CoV-2, the S1 subunit of the S protein of the spike glycoprotein receptor attaches with the ACE2 of the host cell to initiate the attachment process [29]. So, the viral S1—ACE2 blocking cloud is an important checkpoint for inhibiting virus entry into the host cell. Therefore, researchers emphasized nanoparticle-assisted ACE2 receptor and viral S protein blocking through the development of nanoparticles with physical antagonistic features against the ACE2 receptor or S1 protein. Towards this direction, researchers across the globe have targeted the cell–virus adhesion step as an important checkpoint for the inhibition of viral replication and pathogenesis [30]. Several nanoparticles, including Ag NPs, Au NPs, ZnO NPs, CuO NPs, graphene oxide nanoparticles (GO NPs), carbon dots (CDs), as well as lipid and carbohydrate nanoparticles and their nanohybrids, have been suggested and tried out for this inhibition process, as shown in Figure 2. Ag NPs have been found to inhibit the attachment of human immunodeficiency virus-1 (HIV-1) and respiratory syncytial virus (RSV) envelope proteins to the host cell by blocking glycoprotein [30][31]. Several other studies have also reported that some modified Ag NPs with mercaptoethanol sulfonate, tannic acid, and antiviral oseltamivir inhibit the attachment of herpes simplex virus-1 (HSV-1), HSV-2, and influenza virus (H1N1) to the host cell [31][32]. Likewise, Au NPs have been shown to inhibit the attachment of HIV, HSV-1, and H1N1 to lymphocytes, macrophages, and endothelial cells in the brains of mice [33][34][35]. Many oxide nanoparticles, such as CuO NPs, ZnO NPs, and polyglycerol sulfate-coated GO NPs, inhibit the attachment and entry of the hepatitis C virus (HCV), HSV-2, and African swine flu virus into host cells [36][37][38], while carbon nanostructures, like fullerene and CDs, functionalized with boronic acid and 4-aminophenyl boronic acid, inhibit entry of HSV-1, HCoV-229E, H1N1, and porcine epidemic diarrhea virus into the host cell [39][40][41]. Researchers have also applied peptides, polypeptides, and antiviral functionalized nanoparticles, such as β-CD-PACM nanoparticles loaded with acyclovir, hydrophilic N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC), hydrophobically modified HTCC (HM-HTCC), N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium (DOTAP) liposomes, phosphatidylserine (PS) liposomes, phosphatidylcholine (PC) liposomes, polyanionic carbosilane dendrimers, and stearylamine-coated liposome nanoparticles to various viral adhesion processes, and found that these nanohybrids inhibit the attachment of HIV, HSV-1, HSV-2, human coronaviruses HCoV-NL63, and murine hepatitis virus (MHV) to the host cell [42][43][44].
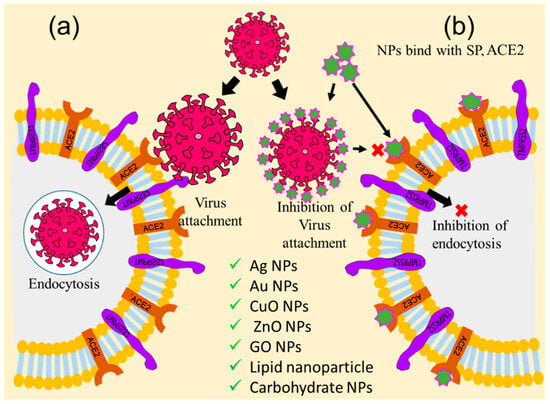
Figure 2. Graphical illustration of the inhibition of receptor-mediated host–virus attachments. (a) Receptor-mediated host–virus attachment and internalization, and (b) nanoparticle-mediated blocking of ACE2 for inhibition of virus attachments (‘×’ indicates inhibition checkpoint for virus attachment).
3.2. Inhibition of Virus Uncoating
Immediately after internalization, the virion coat dissolves due to cytosolic enzyme reactions, resulting in the exposure of the viral genome, which is known as the uncoating stage of viral replication [45]. Uncoating is considered the second most important checkpoint for inhibiting viral replication [45][46]. Therefore, viral encapsulation with nanomaterials could protect the capsid from degradation with cytosolic enzyme activity. Thus, biocompatible viral encapsulating material could be an ideal solution to prevent the multiplication of the virus. Focusing on this, Iron oxide nanoparticles (IONPs), GO NPs, solid-lipid nanoparticles (SLNs), and nano-capsules have been employed to target this checkpoint, inhibit the uncoating process, and thereby impede virus replication [31][47][48]. Figure 3 illustrates these processes. It has been reported that Ag NPs inhibit the uncoating of the Tacaribe virus by blocking its receptor glycoprotein structures [48][49][50][51]. Likewise, carbon-based nanoparticles, such as GO NPs and carbon nanotubes (CNT), have been employed against H1N1 to inhibit uncoating through physical encapsulation of the viral glycoprotein coat, as shown in Figure 3a [52]. Antiviral-coated lipid nanoparticles, for example, nano-capsules entrapped with azidothymidine-triphosphate (AZT-TP), polymers coated with polyethyleneimine, β-cyclodextrin-poly (4-acryloylmorpholine) mono-conjugate (β-CD-PACM), and SLNs loaded with atazanavir, have also been found to inhibit HIV uncoating through targeted delivery of the trapped drug into the cytoplasm [46]. A few recent studies have reported that IO NPs and GO NPs inhibit the uncoating of SARS-CoV-2 through irreversible changes to S1-RBD induced by the formation of IO NP-S1-RBD complexes [47].
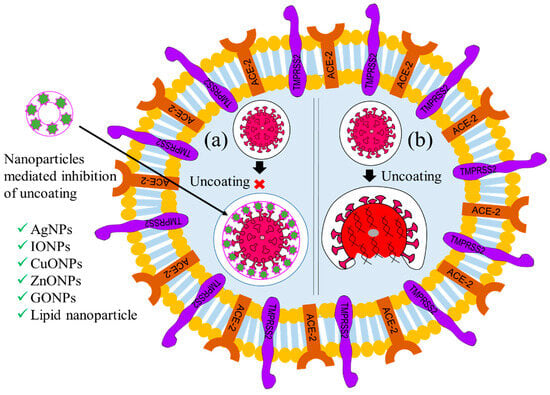
Figure 3. Graphical illustration of the inhibition of the uncoating of a virus. (a) Nanoparticle-mediated inhibition of uncoating and (b) uncoating in absence of NPs. (‘×’ indicates inhibition checkpoint for virus uncoating).
3.3. Inhibition of Viral Gene Expression
Following the uncoating stage, the viral genome replicates to form numerous copies that are later translated into structural and non-structural viral proteins [3][53]. After entry and uncoating, the virus releases its RNA genome in the cytoplasm for gene expression [54]. In this process, positive-sense ssRNA was translated to form ORF-1a and ORF-1b [55]. The ORF was polymerized through RdRp to form dsRNA, and thus, the gene expression occurred. So, inhibition of RdRp-mediated polymerization could be a way to impede gene expression. Thus, this step has also been considered as a checkpoint for inhibiting viral replication. Therefore, nanoparticle-assisted blocking of the RdRp enzyme could also be an ideal choice to prevent viral replication. Several nanoparticles, including CuO NPs, chitosan nanoparticles (Chi NPs), Au NPs, ZnO NPs, GO NPs, Se NPs, CNTs, and CDs have been employed to inhibit viral gene expression at this checkpoint [56][57][58][59], as shown in Figure 4. Studies have reported that CuO NPs inhibit genome expression of Emiliania huxleyi virus 86, HSV, poliovirus, and influenza A through reactive oxygen species (ROS)-mediated oxidative damage of the viral genome [59][60]. Copper nanohybrid particles, such as copper–iodide, gold–copper, and copper–nanostructures, have been used to inhibit HuCoV-229, H1N1, and SARS-CoV viral gene expression; they do so by damaging mRNA and inactivating proteases and polymerase enzymes [57][61]. Likewise, ZnO NPs and polyethylene glycol (PEG)-coated ZnO NPs inhibit H1N1, nidoviruses, and the RNA expression of other viral genes through ROS-mediated inactivation of RNA-dependent RNA polymerase [57][62]. Additionally, Se NPs loaded with siRNA and Chi NPs coated with siRNA inhibit Ebola virus 71 and influenza viral gene expression through targeted delivery of siRNA to the VP1 gene [24][63]. Different carbons and their nanohybrids, such as GO NPs, CDs, CNTs, fullerene derivatives, and quanta dots (QDs), have also been found to inhibit HCV, SARS-CoV, RSV, the pseudorabies virus, the porcine epidemic diarrhea virus, HIV, influenza viruses, and other RNA viruses. Carbon nanohybrids inhibit viral genome replication through activation of IFN-α, alterations of viral proteins, generation of ROS, and inactivation of protease and reverse transcriptase enzymes [61][64]. Furthermore, many antivirus-coated lipid nanoparticles, including PEG-PLGA loaded with V-ATPase, liposomes coated with ivermectin, and solid lipid nanoparticles loaded with adefovir, have been tested at the uncoating checkpoint and found to inhibit H1N1, H3N2 [65], dengue virus, West Nile virus, yellow fever virus [66], and hepatitis B virus (HBV) [67] viral gene expression.
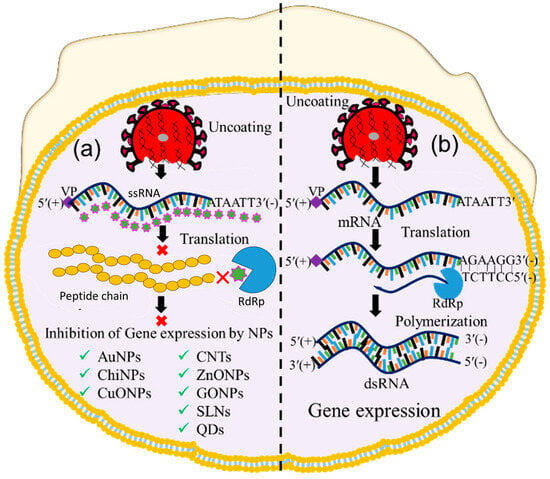
Figure 4. Image showing nanoparticle-assisted inhibition and no inhibition of viral gene expression. (a) Transcription, translation, and polymerization blocking, and (b) gene expression without inhibition. (‘×’ indicates inhibition checkpoint for viral gene expression).
3.4. Inhibition of Protein Synthesis
During gene expression, positive-sense RNA transcript serves as a template for viral protein [68]. The translation of viral transcript is, therefore, considered another vital checkpoint for virus replication [68][69]. Therefore, nanoparticle-assisted inhibition of protein synthesis through blocking ribosomal RNA would also be the ideal choice for preventing virus replication. Bearing this in mind, many researchers used Ag NPs, IO NPs, and Se NPs to inactivate protease and polymerase enzyme-mediated translation processes [69], as illustrated in Figure 5(bi). Furthermore, Ag NPs and polysaccharide-coated Ag NPs have been reported to inhibit glycoprotein synthesis of the Tacaribe and monkeypox viruses [70][71]. Several oxide nanoparticles, including ZnO NPs and IO NPs, have shown similar inhibitory effects on the protein synthesis of H1N1 influenza and HCV by inactivating peroxidase and catalase enzyme activities [72][73], while Se NPs loaded with antiviral drugs also inhibit the protein synthesis of H1N1 [22][46]. Several carbon-based nanoparticles, such as CNTs functionalized with protoporphyrin IX (PPIX) and fullerene derivatives, likewise inhibit the protein synthesis of influenza viruses through RNA degradation and that of HIV by interacting with Vpr, Nef, and Gag proteins [74][75][76]. Additionally, antivirus-coated polymeric nanoparticles such as amantadine-loaded micelles and siRNA-coated PLGA nanoparticles hinder the protein synthesis of H1N1 and HSV-2 by blocking hemagglutinin protein and complementary mRNA strands [77].
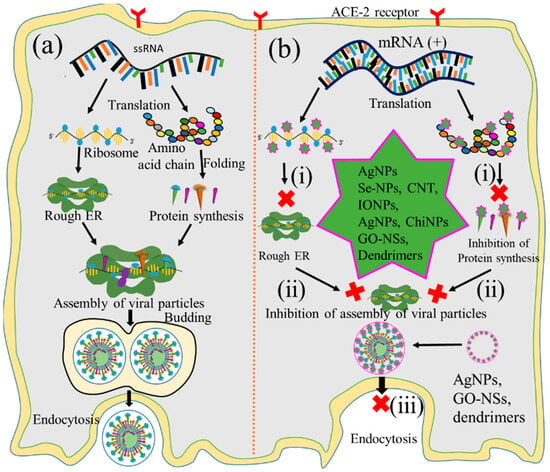
Figure 5. Graphical illustration of the inhibition of protein synthesis. (a) Protein synthesis, viral assembly, and fusion of viral replication and nanoparticle-mediated inhibition of (b): (i) protein synthesis, (ii) viral particle assembly, and (iii) release of virion. (‘×’ indicates inhibition checkpoint for proteib synthesis, Assambly and release of virus).
3.5. Inhibition of the Viral Particle Assembly
The assembly of viral particles involves the transportation of chemically distinct macromolecules through different pathways, such as through interactions between proteins of viral and cellular origin, between viral proteins and nucleic acids, and among viral proteins themselves, to complete the virion. Thus, the assembly of newly synthesized viral protein into the rough endoplasmic reticulum could be another checkpoint for impeding viral replication. Focusing on this, many researchers employed Au NPs, IO NPs, and GO to inhibit such processes at this key checkpoint [78][79][80][81], as illustrated in Figure 5(bii). It has been reported that Au NPs inhibit the assembly of influenza virus, HIV, and HSV proteins, as well as those of other viral particles, by blocking their interactions [82][83]. Likewise, IO NPs coated with poly hexamethylene biguanide inhibit the assembly of HSV-1, viral hemorrhagic septicemia virus, and infectious pancreatic necrosis virus through irreversible damage of viral particles [80], while GO NPs inhibit the porcine epidemic diarrhea and pseudorabies viruses’ protein assemblies through physical interactions between graphene derivatives and virus particles [78][81].
3.6. Inhibition of Virion Release
The last step of the replication pathways is the release of progeny virions, either by lysis of the host cell or through extrusion processes [84]. This step is also considered an important checkpoint for nanoparticle-mediated inhibition of replication to tackle the spread of infectious viruses [85]. Ag NPs, GO NPs, and dendrimers have been applied to inhibit this step by blocking various viral structural proteins [86][87], as shown in Figure 5(biii). Ag NPs inhibit the release of infant HCV extracellular virion through interactions with structural proteins [88]. Likewise, GO NPs inhibit the release of HSV-1 through physical interactions with enveloped proteins [89], and erythrocyte membrane-coated spiky nanostructures impede the release of the influenza A virus by blocking the outer shell of the infant virus [90]. Additionally, dendrimers coated with pentaerythritol derivatives inhibit the release of HIV and enterovirus 71 (EV71) virion by blocking vesicular membranes [91].
4. Scope of Nanotechnology in Controlling Clinical Waves of COVID-19
4.1. Development of Nano-Biosensors
Nano-biosensors are considered an attractive tool worldwide, because they enable the fast and sensitive real-time monitoring of analytes, incorporating biomedical devices that have already been used in the remote monitoring of biophysical parameters such as pulse rates, heart rates, oxygen levels, and pH levels. For example, BIOTEST AG, single-walled carbon nanotubes (SWCNTs), surface plasmon resonance (SPR), plasmonic photothermal (PPT) biosensors, localized surface plasmon resonance (LSPR), surface-enhanced Raman scattering (SERS), and fluorescence-based nano-biosensors have been developed for the detection of HIV, HPV, H1N1, dengue virus, SARS-CoV-2, and other viruses [92].
Wearable devices consisting of multisensory electrodes, including pressure, heat, oxygen, pulse, respiratory, and PH sensors, can measure body parameters such as pulse rates, pressure, or temperatures (Figure 6). Healthcare devices such as sensor patches (e.g., a band-aid adhesive patch used for glucose monitoring [93]), epidermal electronics (e.g., used to detect electrophysiological signals on the epidermis [94][95]), and contact lenses with embedded electronics (such as sensors, transmitters, and amplifiers used for health monitoring [96]) have recently attracted attention because of their potential to be applied in biomedical settings. Additionally, skin-equivalent sensors (such as the SkinEthicTM, Lyon, Franch, MatTek, Ashland, MA 01721, USA, StrataTech, St. Louis, MO, USA) [97][98] or bio-implantable sensors (such as specific absorption rate (SAR)), implantable blood pressure sensors, medical implant communication service (MICS), etc. [99][100]) combined with distance-monitoring devices could be useful for the diagnosis of COVID-19. However, applications of such devices are challenging due to inadequate interactions at the skin–device interface that lead to poor signal acquisition, and they are hence unfit for distance monitoring. Additionally, state-of-the-art devices often exhibit bio-incompatibility issues resulting from adverse tissue reactions, such as erythema, itching, and inflammation, which can cause severe discomfort for users. The signal acquisition efficacy of sensing devices is another challenging aspect that could, however, be tackled through in vitro and in vivo experimentation. The shelf life of such sensors also needs to be determined before they can be applied in real-life settings. Engineering solutions with biocompatible skin equivalent (SE)-embedded multi-electrode sensors that can establish biological communication between the skin and wearables and achieves the signal sensitivity necessary for monitoring clinical parameters of COVID-19 patients from a safe distance are therefore needed. Nano-biosensor-based wearable sensors, skin-equivalent electronics, epidermal electronics, and implantable sensors might be candidates for such distance monitoring devices. Nano-biosensor-based self-monitoring devices could serve as a TTT tool to identify symptomless carries among large populations and reduce horizontal virus transmission. Physicians and other healthcare personnel using such monitoring devices will be able to monitor patients with COVID-19 from a distance without being exposed.
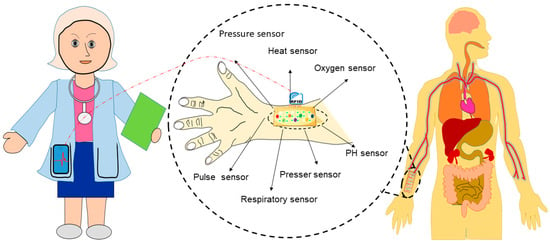
Figure 6. Development of wearable devices for distance monitoring of COVID-19 patients.
4.2. Development of SARS-CoV-2-Neutralizing Nanoparticles
Nanoparticles have been studied in many fields of biomedical sciences for their increased surface area, excellent sensitivity, and enhanced functionality [101][102][103]. They have been suggested as an alternative to antibiotics [104], antifungals [105], and antivirals [106][107] to curb the use of these drugs. The ion exchange ability, enhanced functionality, ion absorption capability, and chemical complexation of multifunctional nanoparticles promise to be effective in neutralizing SARS-CoV-2. However, many nanoparticles exhibit compromised bio-compatibility because of the materials used, underlying chemical synthesis processes, and improper functionalization of target ligands. Nanoparticles from biocompatible, biodegradable, and eco-friendly materials synthesized through green processes could be effective antivirals for controlling the SARS-CoV-2 pandemic.
Nanostructures with RBD-like physical antagonistic features could serve as effective therapeutic agents that block the RBD from the inhibition of ACE2 receptor-mediated cell fusion. NPs functionalized antagonistic nanostructure encapsulated RBD formed that will block the specific RBD site, resulting in the inhibition of RBD-ACE2 adhesion as shown in Figure 7. Mutation-dependent alterations of amino acid residues can then not impact the inhibition process, which may prevent a rapid spread as well as the pathogenicity of variants caused by mutations of the S protein. Considering these differences to previous approaches, nanomaterial-based therapeutics using nanoscale hybrid structures with ACE2 receptor-like antagonism features on their surfaces that can neutralize SARS-CoV-2 well ahead of its adhesion to the ACE2 receptor could serve as an alternative treatment for clinical cases (see Figure 7).
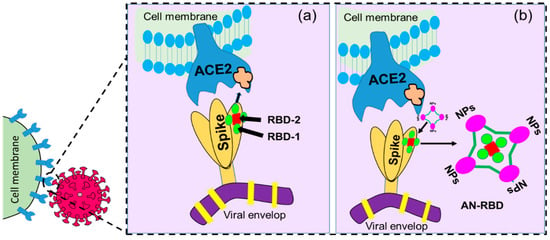
Figure 7. Graphical illustration of (a) receptor-binding domain (RBD)-mediated host cell–virus relationship where RBD-1 is the main part responsible for the binding, and (b) antagonistic nanostructure encapsulated RBD (AN-RBD) inhibiting adhesion.
Figure 8. The proposed scope of COVID-19 treatment approaches: (Step 1) injection of functionalized anti-sialic receptor-like nanoparticles (NPs) and (Step 2) NPs and spike protein (SP) of SARS-CoV-2 attach and block the receptor-binding domain (RBD); SP cannot bind to angiotensin convertase enzyme (ACE2) (‘×’ indicates inhibition checkpoint).
More specifically, nanoparticles functionalized with anti-salicylic acid will be effective in neutralizing viruses circulating in a multicellular host and thus prevent further disease progression (Figure 8). After entering the circulation, anti-salicylic acid-functionalized nanoparticles will be coupled with viruses to inhibit their attachment to the ACE2 receptor on the cell surface, which will then inhibit the fusion process. This means that the ACE2-activated angiotensin regulation mechanism will remain uninterrupted, and homeostasis will be maintained in the cardiovascular system. Nano-biosensor-based early detection of infections will also enable nanoparticle-assisted neutralization of SARS-CoV-2 during primary viremia and thus prevent fatalities.
4.3. Development of Nanoscale Antiviral Drugs and Vaccine Carriers
A number of drug nanocarriers have been introduced for other purposes, for example, lipid-based nanocarriers for targeted therapies [108], RNA and protein nanocarriers for cancer and cellular niche therapy [24]. In the same way, tissue-specific drug carriers can be developed for the delivery of drugs against COVID-19 (Figure 9). Patients experiencing respiratory distress can be treated with a nanocarrier probed for lung tissue, while one probed for renal tissue or the cardiovascular system can be employed for patients experiencing renal or cardiovascular dysfunction. Such targeted delivery avoids not only unwanted exposure of unaffected systems to antivirals but also side effects.
Figure 9. The suggested approach of targeted delivery of nanoparticles: (a) functionalized NPs will be conjugated to the drug or vaccine carrier, (b) drug-capped NPs will be carried to the targeted cells, and (c) ACE2 receptor of the most vulnerable lung cell will be blocked by the drug-capped NPs, which represents the nano-preventive approach for neutralizing virus in the viremia stage (‘×’ indicates inhibition checkpoint).
This entry is adapted from the peer-reviewed paper 10.3390/ijms241713130
References
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790.
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424.
- Bakhshandeh, B.; Jahanafrooz, Z.; Abbasi, A.; Goli, M.B.; Sadeghi, M.; Mottaqi, M.S.; Zamani, M. Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microb. Pathog. 2021, 154, 104831.
- O’Toole, Á.; Pybus, O.G.; Abram, M.E.; Kelly, E.J.; Rambaut, A. Pango lineage designation and assignment using SARS-CoV-2 spike gene nucleotide sequences. BMC Genom. 2022, 23, 121.
- McGill, J.L.; Kelly, S.M.; Kumar, P.; Speckhart, S.; Haughney, S.L.; Henningson, J.; Narasimhan, B.; Sacco, R.E. Efficacy of mucosal polyanhydride nanovaccine against respiratory syncytial virus infection in the neonatal calf. Sci. Rep. 2018, 8, 3021.
- Qi, M.; Zhang, X.E.; Sun, X.; Zhang, X.; Yao, Y.; Liu, S.; Chen, Z.; Li, W.; Zhang, Z.; Chen, J.; et al. Intranasal Nanovaccine Confers Homo- and Hetero-Subtypic Influenza Protection. Small 2018, 14, e1703207.
- Vicente, S.; Diaz-Freitas, B.; Peleteiro, M.; Sanchez, A.; Pascual, D.W.; Gonzalez-Fernandez, A.; Alonso, M.J. A Polymer/Oil Based Nanovaccine as a Single-Dose Immunization Approach. PLoS ONE 2013, 8, e62500.
- Focosi, D.; Novazzi, F.; Genoni, A.; Dentali, F.; Gasperina, D.D.; Baj, A.; Maggi, F. Emergence of SARS-CoV-2 Spike Protein Escape Mutation Q493R after Treatment for COVID-19. Emerg. Infect. Dis. 2021, 27, 17–20.
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091.
- Vijayan, V.; Mohapatra, A.; Uthaman, S.; Park, I.K. Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics 2019, 11, 534.
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg. Microbes Infect. 2022, 11, 1–5.
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661.
- Rahman, A.; Rasid, H.; Ali, I.; Yeachin, N.; Alam, S.; Hossain, K.S.; Kaf, A. Facile Synthesis and Application of Ag-NPs for Controlling Antibiotic-Resistant Pseudomonas spp. and Bacillus spp. in a Poultry Farm Environment. J. Nanotechnol. 2023, 2023, 6260066.
- Khan, I.; Khan, M.; Umar, M.N.; Oh, D.H. Nanobiotechnology and its applications in drug delivery system: A review. IET Nanobiotechnol. 2015, 9, 396–400.
- Kafi, M.A.; Kim, T.H.; Yagati, A.K.; Kim, H.; Choi, J.W. Nanoscale fabrication of a peptide layer in cell chip to detect effects of environmental toxins on HEK293 cells. Biotechnol. Lett. 2010, 32, 1797–1802.
- Kafi, M.A.; Cho, H.Y.; Choi, J.W. Engineered peptide-based nanobiomaterials for electrochemical cell chip. Nano Converg. 2016, 3, 17.
- Kafi, M.A.; Kim, T.H.; Yea, C.H.; Kim, H.; Choi, J.W. Effects of nanopatterned RGD peptide layer on electrochemical detection of neural cell chip. Biosens. Bioelectron. 2010, 26, 1359–1365.
- Kafi, M.A.; Cho, H.Y.; Choi, J.W. Neural cell chip based electrochemical detection of nanotoxicity. Nanomaterials 2015, 5, 1181–1199.
- Rani Sarkar, M.; Rashid, M.H.; Rahman, A.; Kafi, M.A.; Hosen, M.I.; Rahman, M.S.; Khan, M.N. Recent advances in nanomaterials based sustainable agriculture: An overview. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100687.
- Wong, K.V. Nanoscience and Nanotechnology. In Nanotechnology and Energy; Jenny Stanford Publishing: New York, NY, USA, 2018; ISBN 9781259007323.
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778.
- Weiss, C.; Carriere, M.; Fusco, L.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Pasquali, M.; Pasquali, M.; Scott, J.A.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406.
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143.
- Vahedifard, F.; Chakravarthy, K. Nanomedicine for COVID-19: The role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater. 2021, 4, 75–99.
- Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.O.; Pottier, A.; Schi, R.; Ceccaldi, A.; Schmid, R. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company ’s public news and information. J. Control. Release 2020, 326, 164–171.
- Umair, M.; Javed, I.; Rehman, M.; Madni, A.; Javeed, A.; Ghafoor, A.; Ashraf, M. Nanotoxicity of inert materials: The case of gold, silver and iron. J. Pharm. Pharm. Sci. 2016, 19, 161–180.
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Front. Pharmacol. 2020, 11, 1189.
- Aderibigbe, B.A. Metal-based nanoparticles for the treatment of infectious diseases. Molecules 2017, 22, 1370.
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the inhibition of viral infections. Molecules 2015, 20, 14051–14081.
- Mohammed Fayaz, A.; Ao, Z.; Girilal, M.; Chen, L.; Xiao, X.; Kalaichelvan, P.T.; Yao, X. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: A new approach to inhibit HIV- and HSV-transmitted infection. Int. J. Nanomed. 2012, 7, 5007–5018.
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 2009, 20, 1497–1502.
- Vijayakumar, S.; Ganesan, S. Gold Nanoparticles as an HIV Entry Inhibitor. Curr. HIV Res. 2014, 10, 643–646.
- Kesarkar, R. L-Cysteine Functionalized Gold Nanocargos Potentiates Anti-HIV Activity of Azidothymydine against HIV-1Ba-L Virus. Juniper Online J. Immuno Virol. 2015, 1, 555552.
- Mishra, Y.K.; Adelung, R.; Röhl, C.; Shukla, D.; Spors, F.; Tiwari, V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antivir. Res. 2011, 92, 305–312.
- Shoji, M.; Takahashi, E.; Hatakeyama, D.; Iwai, Y.; Morita, Y.; Shirayama, R.; Echigo, N.; Kido, H.; Nakamura, S.; Mashino, T.; et al. Anti-Influenza Activity of C60 Fullerene Derivatives. PLoS ONE 2013, 8, e66337.
- Ziem, B.; Rahn, J.; Donskyi, I.; Silberreis, K.; Cuellar, L.; Dernedde, J.; Keil, G.; Mettenleiter, T.C.; Haag, R. Polyvalent 2D Entry Inhibitors for Pseudorabies and African Swine Fever Virus. Macromol. Biosci. 2017, 17, 1600499.
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon N. Y. 2016, 110, 278–285.
- Łoczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974.
- Tahara, K.; Kobayashi, M.; Yoshida, S.; Onodera, R.; Inoue, N.; Takeuchi, H. Effects of cationic liposomes with stearylamine against virus infection. Int. J. Pharm. 2018, 543, 311–317.
- Tiwari, V.; Liu, J.; Valyi-Nagy, T.; Shukla, D. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J. Biol. Chem. 2011, 286, 25406–25415.
- Figueroa, S.M.; Veser, A.; Abstiens, K.; Fleischmann, D.; Beck, S.; Goepferich, A. Influenza A virus mimetic nanoparticles trigger selective cell uptake. Proc. Natl. Acad. Sci. USA 2019, 116, 9831–9836.
- Cavalli, R.; Donalisio, M.; Civra, A.; Ferruti, P.; Ranucci, E.; Trotta, F.; Lembo, D. Enhanced antiviral activity of Acyclovir loaded into β-cyclodextrin-poly(4-acryloylmorpholine) conjugate nanoparticles. J. Control. Release 2009, 137, 116–122.
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25.
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2020, 11, 748–787.
- Lategan, K.; Alghadi, H.; Bayati, M.; de Cortalezzi, M.F.; Pool, E. Effects of graphene oxide nanoparticles on the immune system biomarkers produced by RAW 264.7 and human whole blood cell cultures. Nanomaterials 2018, 8, 125.
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 19.
- Trigilio, J.; Antoine, T.E.; Paulowicz, I.; Mishra, Y.K.; Adelung, R.; Shukla, D. Tin Oxide Nanowires Suppress Herpes Simplex Virus-1 Entry and Cell-to-Cell Membrane Fusion. PLoS ONE 2012, 7, e48147.
- Khandelwal, N.; Kaur, G.; Kumar, N.; Tiwari, A. Application of silver nanoparticles in viral inhibition: A new hope for antivirals. Dig. J. Nanomater. Biostruct. 2014, 9, 175–186.
- Soiza, R.L.; Donaldson, A.I.C.; Myint, P.K. Vaccine against arteriosclerosis: An update. Ther. Adv. Vaccines 2018, 9, 259–261.
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C 2020, 112, 110924.
- Villanueva, M.T. Interfering viral-like particles inhibit SARS-CoV-2 replication. Nat. Rev. Drug Discov. 2022, 21, 19.
- Ishida, N. Laboratory diagnosis of virus diseases. Boei. Eisei. 1962, 9, 330–333.
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170.
- Báez-Santos, Y.M.; John, S.E.S.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015, 115, 21–38.
- Ishida, T. Review on The Role of Zn2+ Ions in Viral Pathogenesis and the Effect of Zn2+ Ions for Host Cell-Virus Growth Inhibition. Am. J. Biomed. Sci. Res. 2019, 2, 28–37.
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251.
- Sagripanti, J.L.; Routson, L.B.; Lytle, C.D. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl. Environ. Microbiol. 1993, 59, 4374–4376.
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750.
- te Velthuis, A.J.W.; van den Worml, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176.
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70.
- Reynolds, N.; Dearnley, M.; Hinton, T.M. Polymers in the Delivery of siRNA for the Treatment of Virus Infections; Springer International Publishing: New York, NY, USA, 2017; Volume 375, ISBN 4106101701276.
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459.
- Hu, C.M.J.; Chen, Y.T.; Fang, Z.S.; Chang, W.S.; Chen, H.W. Antiviral efficacy of nanoparticulate vacuolar ATPase Inhibitors Against Influenza virus infection. Int. J. Nanomed. 2018, 13, 8579–8593.
- Croci, R.; Bottaro, E.; Chan, K.W.K.; Watanabe, S.; Pezzullo, M.; Mastrangelo, E.; Nastruzzi, C. Liposomal Systems as Nanocarriers for the Antiviral Agent Ivermectin. Int. J. Biomater. 2016, 2016, 8043983.
- Xing-Guo, Z.; Jing, M.; Min-Wei, L.; Sai-Ping, J.; Fu-Qiang, H.; Yong-Zhong, D. Solid lipid nanoparticles loading adefovir dipivoxil for antiviral therapy. J. Zhejiang Univ. Sci. B 2008, 9, 506–510.
- DeDiego, M.L.; Nieto-Torres, J.L.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Usera, F.; Enjuanes, L. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014, 194, 124–137.
- Raman, R.; Patel, K.J.; Ranjan, K. COVID-19: Unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules 2021, 11, 993.
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133.
- López, N.; Jácamo, R.; Franze-Fernández, M.T. Transcription and RNA Replication of Tacaribe Virus Genome and Antigenome Analogs Require N and L Proteins: Z Protein Is an Inhibitor of These Processes. J. Virol. 2001, 75, 12241–12251.
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Bhawna; Madhukar, M.; et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329.
- Ramphul, K.; Mejias, S.G. Coronavirus Disease: A Review of a New Threat to Public Health. Cureus 2020, 20, 2019–2020.
- Krishnaraj, R.; Chandran, S.; Pal, P.; Berchmans, S. Investigations on the Antiretroviral Activity of Carbon Nanotubes Using Computational Molecular Approach. Comb. Chem. High Throughput Screen. 2014, 17, 531–535.
- Martinez, Z.S.; Castro, E.; Seong, C.S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741.
- Rosen, Y.; Gurman, P. Carbon Nanotubes for Drug Delivery Applications. In Nanotechnology and Drug Delivery; CRC Press: Boca Raton, FL, USA, 2014; pp. 233–248.
- Steinbach, J.M.; Weller, C.E.; Booth, C.J.; Saltzman, W.M. Polymer nanoparticles encapsulating siRNA for treatment of HSV-2 genital infection. J. Control. Release 2012, 162, 102–110.
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21578–21579.
- Cagno, V.; Andreozzi, P.; D’alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Goffic, R.L.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195–203.
- Bromberg, L.; Bromberg, D.J.; Hatton, T.A.; Bandín, I.; Concheiro, A.; Alvarez-Lorenzo, C. Antiviral properties of polymeric aziridine- and biguanide-modified core-shell magnetic nanoparticles. Langmuir 2012, 28, 4548–4558.
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193.
- Ghaffari, E.; Rezatofighi, S.E.; Ardakani, M.R.; Rastegarzadeh, S. Delivery of antisense peptide nucleic acid by gold nanoparticles for the inhibition of virus replication. Nanomedicine 2019, 14, 1827–1840.
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 Attachment, Entry, and Cell-to-Cell Spread by Functionalized Multivalent Gold Nanoparticles. Small 2010, 6, 1044–1050.
- Zhand, S.; Jazi, M.S.; Mohammadi, S.; Rasekhi, R.T.; Rostamian, G.; Kalani, M.R.; Rostamian, A.; George, J.; Douglas, M.W. COVID-19: The immune responses and clinical therapy candidates. Int. J. Mol. Sci. 2020, 21, 5559.
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868.
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103–4114.
- Maduray, K.; Parboosing, R. Metal Nanoparticles: A Promising Treatment for Viral and Arboviral Infections. Biol. Trace Elem. Res. 2020, 199, 3159–3176.
- Zhou, T.; Guo, H.; Guo, J.T.; Cuconati, A.; Mehta, A.; Block, T.M. Hepatitis B virus e antigen production is dependent upon covalently closed circular (ccc) DNA in HepAD38 cell cultures and may serve as a cccDNA surrogate in antiviral screening assays. Antivir. Res. 2006, 72, 116–124.
- Sametband, M.; Kalt, I.; Gedanken, A.; Sarid, R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 1228–1235.
- Ren, S.; Fraser, K.; Kuo, L.; Chauhan, N.; Adrian, A.T.; Zhang, F.; Linhardt, R.J.; Kwon, P.S.; Wang, X. Designer DNA nanostructures for viral inhibition. Nat. Protoc. 2022, 17, 282–326.
- Martínez-Gualda, B.; Sun, L.; Martí-Marí, O.; Mirabelli, C.; Delang, L.; Neyts, J.; Schols, D.; Camarasa, M.J.; San-Félix, A. Modifications in the branched arms of a class of dual inhibitors of HIV and EV71 replication expand their antiviral spectrum. Antivir. Res. 2019, 168, 210–214.
- Shand, H.; Dutta, S.; Rajakumar, S.; James Paulraj, S.; Mandal, A.K.; KT, R.D.; Ghorai, S. New Age Detection of Viruses: The Nano-Biosensors. Front. Nanotechnol. 2022, 3, 814550.
- Günl, F.; Mecate-zambrano, A.; Rehländer, S.; Hinse, S.; Ludwig, S.; Brunotte, L. Shooting at a Moving Target—Effectiveness and Emerging Challenges for SARS-CoV-2 Vaccine Development. Vaccines 2021, 9, 1052.
- Simion, V.; Stan, D.; Constantinescu, C.A.; Deleanu, M.; Dragan, E.; Tucureanu, M.M.; Gan, A.M.; Butoi, E.; Constantin, A.; Manduteanu, I.; et al. Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J. Pharm. Pharmacol. 2016, 68, 195–207.
- Cho, E.; Mohammadifar, M.; Choi, S. A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines 2017, 8, 265.
- Kim, J.H.; Kim, S.R.; Kil, H.J.; Kim, Y.C.; Park, J.W. Highly Conformable, Transparent Electrodes for Epidermal Electronics. Nano Lett. 2018, 18, 4531–4540.
- Baldassi, D.; Ambike, S.; Feuerherd, M.; Cheng, C.C.; Peeler, D.J.; Feldmann, D.P.; Porras-Gonzalez, D.L.; Wei, X.; Keller, L.A.; Kneidinger, N.; et al. Inhibition of SARS-CoV-2 replication in the lung with siRNA/VIPER polyplexes. J. Control. Release 2022, 345, 661–674.
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41.
- Mutashar, S.; Hannan, M.A.; Samad, S.A.; Hussain, A. Analysis and optimization of spiral circular inductive coupling link for bio-implanted applications on air and within human tissue. Sensors 2014, 14, 11522–11541.
- Fang, Q.; Lee, S.Y.; Permana, H.; Ghorbani, K.; Cosic, I. Developing a wireless implantable body sensor network in MICS band. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 567–576.
- Rahman, A.; Roy, K.J.; Rahman, K.M.A.; Aktar, M.K.; Kafi, M.A.; Islam, M.S.; Rahman, M.B.; Islam, M.R.; Hossain, K.S.; Rahman, M.M.; et al. Adhesion and proliferation of living cell on surface functionalized with glycine nanostructures. Nano Sel. 2021, 2, 188–200.
- Rajan, V.; Sivaraman, G.K.; Vijayan, A.; Elangovan, R.; Prendiville, A.; Bachmann, T.T. Genotypes and phenotypes of methicillin-resistant staphylococci isolated from shrimp aquaculture farms. Environ. Microbiol. Rep. 2022, 14, 391–399.
- Kafi, M.A.; Aktar, M.K.; Heidari, H. Mammalian Cell-Based Electrochemical Sensor for Label-Free Monitoring of Analytes. Smart Sens. Environ. Med. Appl. 2020, 9, 43–60.
- Roy, K.J.; Rahman, A.; Hossain, K.; Rahman, B. Antibacterial Investigation of Silver Nanoparticle against Staphylococcus, E. coli and Salmonella Isolated from Selected Live Bird Markets. Appl. Microbiol. Open Access 2020, 6, 173.
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32.
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918.
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V.; et al. Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021.
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta—Rev. Cancer 2014, 1846, 75–87.
This entry is offline, you can click here to edit this entry!
