Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Rheumatology
Hyaluronic acid (HA) is as naturally occurring glycosaminoglycan composed of repeating disaccharide units consisting of glucuronic acids and N-acetylglucosamine, resulting in different molecular weights. HA plays a crucial pathophysiological role in rheumatic diseases, especially concerning joint health and function.
- hyaluronic acid
- rheumatology
- osteoarthritis
- rheumatoid arthritis
- joint lubrication
1. Introduction
Hyaluronic acid (HA) plays an important role in a wide range of medical physiological and pathological conditions: Notably, it finds application in dermatology, ophthalmology, cosmetic medicine, and rheumatology [1,2,3]. Its significance extends to wound healing, granulation, and cell migration [4]. However, the efficacy of HA in rheumatology remains a subject of controversy at times [5,6].
HA is as naturally occurring glycosaminoglycan composed of repeating disaccharide units consisting of glucuronic acids and N-acetylglucosamine (Figure 1), resulting in different molecular weights [7,8,9]. This structural variability imparts diverse functional implications in both physiological and pathological contexts [10]. HA is commercially produced through extraction from animal tissues, such as chicken combs, and from Streptococci bacteria [11].
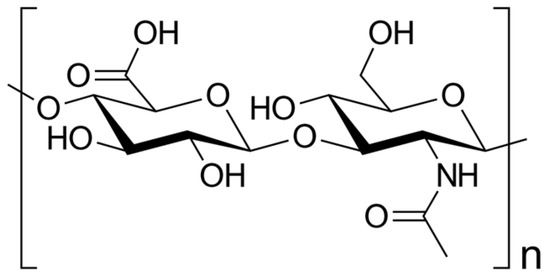
Figure 1. Skeletal formula of hyaluronan—a polymer consisting of D-glucuronic acid and N-acetyl-D-glucosamine linked via alternating β-(1→4) and β-(1→3) glycosidic bonds [7].
Functionally, HA demonstrates remarkable water-binding capacity, rendering it an essential constituent of the extracellular matrix (ECM) [12]. Its elongated, unbranched chains create a gel-like network, imparting hydration and lubrication to crucial tissues like the skin, cartilage, and synovial fluid [13,14,15].
In the field of rheumatology, HA has garnered substantial attention, owing to its pivotal involvement in joint health and its relevance to diseases like osteoarthritis (OA) and rheumatoid arthritis (RA) [16,17].
OA is a degenerative joint disorder characterized by the gradual deterioration of articular cartilage, resulting in pain, stiffness, and diminished joint function [18]. There is no cure for OA, so doctors usually treat OA symptoms with a combination of therapies [18]. HA serves as a lubricant and shock absorber within the synovial fluid, facilitating smooth joint movements [19]. However, in OA, the concentration and quality of HA decrease, compromising its protective and viscoelastic properties due to heightened degradation and decreased synthesis. Consequently, this leads to impaired cartilage function and joint degeneration [20]. As a therapeutic approach, supplementation with exogenous HA has emerged to alleviate symptoms and enhance joint function in OA patients [21]. By restoring synovial fluid viscosity and promoting cartilage repair, HA aids in improving joint mobility and reducing pain [16].
On the other hand, RA is an autoimmune and inflammatory disease characterized by the immune system mistakenly attacking healthy cells, resulting in inflammation, particularly in the joints, leading to painful swelling [22]. RA can be effectively treated and managed with medication(s) and self-management strategies [22]. In RA, the level of HA in the synovial fluid is significantly diminished, causing reduced lubrication and increased inflammation and pain [23].
Numerous studies have explored the potential therapeutic benefits of exogenous HA supplementation in rheumatic diseases. Through intra-articular injections, HA has shown promise in improving joint mobility, reducing pain, and promoting cartilage repair by restoring synovial fluid viscosity [24].
Furthermore, HA has demonstrated immunomodulatory effects, including the suppression of pro-inflammatory cytokines and the promotion of anti-inflammatory cytokine production [25]. This suggests that HA may also hold promise in the treatment of immune-mediated rheumatic diseases [26].
Moreover, researchers have explored HA’s potential applications in drug delivery systems and tissue engineering due to its biocompatibility and biodegradability [27].
However, the use of HA therapy in rheumatology remains a topic of controversy, with conflicting evidence regarding its efficacy and safety [5,28].
In summary, HA represents a promising therapeutic option in the field of rheumatology due to its potential to enhance joint function and alleviate inflammation and pain [29]. Nevertheless, further investigation is required to fully elucidate its therapeutic potential in rheumatic diseases.
2. Hyaluronic Acid: Structure, Function, and Biochemistry
HA plays a crucial role in diverse cellular and tissue processes, encompassing hydration, lubrication, tissue repair, regulation of inflammation, and cell signaling [14]. It is naturally synthesized by various cell types, predominantly fibroblasts, chondrocytes, and synoviocytes [30]. The biosynthesis of HA takes place in the plasma membrane through the coordinated activity of specific enzymes, including hyaluronan synthases [30].
2.1. Molecular Structure of Hyaluronic Acid
Hyaluronan synthases catalyze the addition of glucuronic acid and N-acetylglucosamine, leading to the formation of the repeating disaccharide units that constitute HA [31]. The molecular weight of HA displays highly variability, ranging from several hundred kilo Da to millions of kilo Da, exerting a direct impact on its functional properties [32]: Notably, higher-molecular-weight HA exhibits increased viscosity [33], thereby affecting its flow and lubrication capability, i.e., in joints [34]. As a result, high-molecular-weight HA provides superior lubrication and cushioning effects [35].
2.2. Biosynthesis and Degradation of Hyaluronic Acid
HA turnover in tissues is intricately regulated by a delicate balance between biosynthesis and degradation processes [36]. The degradation of HA primarily involves the action of enzymes known as hyaluronidases, which cleave HA into smaller fragments [37]. Hyaluronidase enzymes are categorized into several families, including HYAL1, HYAL2, and PH-20 [38], and they play a pivotal role in maintaining the appropriate concentration and size distribution of HA within tissues [39]. Furthermore, the degradation of HA can be modulated by reactive oxygen species, matrix metalloproteinases (MMPs), and other factors present in the extracellular environment [40].
2.3. Physiological Functions and Distribution in Tissues of Hyaluronic Acid
HA plays a critical role in tissue repair and remodeling processes within the human body [41]. It participates in various stages of wound healing, encompassing inflammation, cell migration, proliferation, and ECM remodeling [42]. As a scaffolding molecule, HA provides essential structural support and aids in cell migration during tissue repair [43] (Table 1).
Table 1. Physiological functions of hyaluronic acid (HA) in rheumatology.
| Function | Mechanisms | References |
|---|---|---|
| Lubrication of joints | HA provides lubrication and viscoelastic properties to synovial fluid, reducing friction between joint surfaces and enhancing joint mobility. | [44] |
| Chondroprotection | HA exhibits chondroprotective effects by promoting cartilage matrix synthesis, reducing matrix degradation, and inhibiting the activity of proteolytic enzymes | [45,46] |
| Anti-inflammatory activity | HA can modulate inflammation by reducing the expression of pro-inflammatory cytokines and enzymes, inhibiting leukocyte migration, and suppressing immune responses. | [47,48] |
| Tissue repair and remodeling | HA plays a role in tissue repair and remodeling processes by promoting cell migration, angiogenesis, and extracellular matrix remodeling | [44,49] |
| Viscoelasticity | HA contributes to the viscoelastic properties of connective tissues, maintaining tissue integrity, elasticity, and shock-absorbing capabilities. | [48,50] |
Selected studies on the physiological role of HA in rheumatology. This is not an exhaustive list, and further research and clinical trials have been conducted in this field. For more detailed information, it is recommended to refer to the referenced papers.
During the inflammatory phase of wound healing, HA is involved in the recruitment and activation of immune cells, such as macrophages and neutrophils [51]. HA fragments generated during tissue injury can function as damage-associated molecular patterns and trigger immune responses [51]. Moreover, HA promotes the infiltration of immune cells into the wound site, facilitating the removal of debris and pathogens [52]. In the subsequent proliferative phase, HA contributes to cell migration and proliferation [53]. It forms a provisional matrix that guides cell movement and stimulates cell proliferation [53]. HA receptors, such as CD44 and RHAMM, mediate cell adhesion, migration, and signal transduction, enabling cells to migrate into the wound area and contribute to tissue repair [54]. Furthermore, HA plays a regulatory role in ECM remodeling during tissue repair [55]. It interacts with other components, such as fibronectin and collagen, promoting their assembly and organization [56]. HA also influences the activity of enzymes involved in ECM remodeling, such as MMPs and tissue inhibitors of MMPs, which are essential for matrix turnover and remodeling [57].
Beyond its involvement in tissue repair, HA also plays a role in tissue remodeling processes, such as embryonic development, organ morphogenesis, and angiogenesis [58]. HA provides a structural framework for cell migration and tissue organization during these processes [59]. It regulates cell behavior, including cell differentiation, proliferation, and survival, through interactions with specific receptors and signaling pathways [60].
Overall, HA exhibits a multifaceted role in tissue repair and remodeling, contributing to inflammation resolution, cell migration, proliferation, and ECM (re)organization [61]. Its involvement in these processes highlights its significance in wound healing, tissue regeneration, and developmental biology [62].
Regarding tissue distribution, HA is widely distributed throughout the body, with particularly high concentrations found in connective tissues, such as the skin, synovial fluid, and cartilage [63]. In the skin, HA resides in the ECM and contributes to tissue hydration, elasticity, and wound healing [64]. In the synovial fluid of joints, HA forms a viscous, gel-like substance that provides lubrication, shock absorption, and nutrient supply to the articular cartilage [65]. Additionally, HA is present in the vitreous humor of the eye, where it helps maintain the transparency and shape of the eyeball [66]. Moreover, HA is found in other tissues, including the umbilical cord, umbilical vessels, and embryonic tissues, where it plays crucial roles in development and tissue morphogenesis [67].
3. Pathophysiological Role of Hyaluronic Acid in Rheumatic Diseases
HA plays a crucial pathophysiological role in rheumatic diseases, especially concerning joint health and function [68]. In a healthy joint, HA’s viscoelastic properties facilitate smooth movement of the joint surfaces and shield the cartilage from excessive mechanical stress [69]. HA serves as a shock absorber, mitigating the impact on the joint and thereby reducing the risk of damage. However, in rheumatic conditions such as OA and RA, significant alterations occur in the HA metabolism and distribution within the joint [70].
3.1. Osteoarthritis
3.1.1. Role of Hyaluronic Acid in Joint Lubrication
In OA, there is a notable reduction in the concentration of HA within the synovial fluid, leading to diminished joint lubrication and compromised cartilage protection [69]. This decline in HA levels can be attributed to an imbalance between HA synthesis and degradation, with increased activity of hyaluronidases, the enzymes responsible for HA breakdown [71]. Consequently, the cartilage becomes vulnerable to wear and tear, resulting in pain, stiffness, and functional impairment [72]. Dysregulated HA metabolism, including increased synthesis and degradation, contributes to cartilage degradation, synovitis, and pain in OA [73]. The altered biomechanical properties of HA affect joint lubrication, chondrocyte activity, and inflammation, further exacerbating disease progression [74].
3.1.2. Protective Effects on Chondrocytes and Cartilage Matrix
HA serves as a fundamental component of the cartilage matrix, playing a crucial role in maintaining joint integrity. However, in OA, changes occur in the quantity, molecular weight, and distribution of HA, which can impact its functional properties [75]. The molecular weight of HA influences its retention time within the tissues [76]. Generally, higher-molecular-weight HA exhibits a longer half-life, meaning it remains in the joint or tissue for a more extended period before being broken down and cleared by the body [8]. This prolonged presence may result in longer-lasting effects [8]. On the other hand, lower-molecular-weight HA can more easily penetrate the ECM and reach target cells, while higher-molecular-weight HA may have more limited diffusion [8].
3.1.3. Modulation of Inflammation and Synovial Fluid Changes
Inflammation is a hallmark of rheumatic diseases [77], and HA plays a multifaceted role in this process [78]. Pathological conditions often lead to changes in the quantity and molecular weight of HA in synovial fluid, with implications for disease severity and progression [79]. During the inflammatory phase, HA actively participates in the recruitment and activation of immune cells, such as macrophages and neutrophils [79]. HA engages with specific cell surface receptors, such as CD44 [80], and the binding affinity of HA to these receptors can vary depending on its molecular weight [81]. Distinct fractions of HA, characterized by different molecular weights, can have specific effects on cell signaling, inflammation, and tissue repair processes [82].
3.2. Rheumatoid Arthritis
In RA, the inflammatory process significantly disrupts HA homeostasis [83]. Synovial inflammation induces the release of pro-inflammatory cytokines and enzymes that promote the degradation of HA, leading to a decrease in its concentration and alterations in its molecular weight distribution [84]. These changes in HA metabolism have profound effects on joint lubrication, exacerbate cartilage damage, and perpetuate the inflammatory cycle [85].
3.2.1. Altered Hyaluronic Acid Synthesis and Breakdown
In RA, alterations in HA synthesis and breakdown contribute to the pathogenesis of the disease [70]. Synovial fibroblasts, key players in RA pathophysiology, exhibit dysregulated HA synthesis, leading to increased production and accumulation of HA in the synovial fluid and tissues [86]. This abnormal HA synthesis is influenced by various factors, including pro-inflammatory cytokines and growth factors, which stimulate the expression of HA synthases [87]. Concurrently, increased HA degradation occurs due to upregulated expression and activity of hyaluronidases [88]. The imbalance between HA synthesis and degradation results in the accumulation of fragmented HA in the synovial fluid, exacerbating inflammation and joint damage [89]. Moreover, the presence of HA fragments in the synovium further amplifies the inflammatory response by activating immune cells and promoting the production of pro-inflammatory mediators [90]. The presence of HA fragments and their interaction with CD44 receptors contribute to the perpetuation of chronic inflammation and joint damage in RA [91].
3.2.2. Inflammatory Mediator Modulation
Moreover, the anti-inflammatory properties of HA, which typically involve the modulation of the inflammatory responses within the joints, are disturbed in RA [83]. The inhibitory action of HA on the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-1 beta, and the suppression of inflammatory enzymes, such as cyclooxygenase-2 and MMPs, become dysregulated [92]. During the subsequent proliferative phase, HA continues to play a role in cell migration and proliferation, forming a provisional matrix that guides cell movement and stimulates cell proliferation [93].
3.2.3. Effects on Synovial Hyperplasia and Pannus Formation
HA exerts significant effects on synovial hyperplasia and pannus formation in RA [93]. It plays a pivotal role in promoting synovial hyperplasia by stimulating the proliferation and migration of synovial fibroblasts [94]. Furthermore, it interacts with CD44 receptors on the surface of these fibroblasts, triggering intracellular signaling cascades that promote cell survival, proliferation, and ECM production [95]. Additionally, HA enhances the expression of various pro-inflammatory mediators, such as cytokines and chemokines, further driving synovial hyperplasia and inflammation [96]. Additionally, HA fragments, generated due to the increased breakdown of HA in RA, can stimulate the production of matrix-degrading enzymes, leading to cartilage and bone destruction [97].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15092247
This entry is offline, you can click here to edit this entry!
