Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Radiation therapy is an important part of conservative breast cancer treatment. Boost radiation of the tumor bed at enough doses is often necessary to increase local control of the disease. There are several techniques for administering a boost, including intraoperative radiotherapy.
- breast cancer
- whole breast irradiation
- intraoperative radiotherapy
1. Introduction
Radical mastectomy was the treatment of choice for breast cancer patients in the 20th century but in the nineteen-seventies it was replaced by breast-conserving surgery followed by adjuvant breast irradiation and this is now the standard option to treat patients with localized breast cancer [1][2]. Conservative treatment reaches similar results in terms of survival as mastectomy, or sometimes better [3].
Historically, breast radiotherapy consisted of whole breast irradiation (WBI) administering 48–50 Gy at a classical fractionation of 1.8–2 Gy doses. These classical treatment schedules can be replaced by daily hypofractionated schedules, administering 15–16 fractions between 2.66 Gy and 2.85 Gy [4][5]. After evidence reached by randomized trials, moderate hypofractionation obtained similar results in terms of local control, tolerance and cosmesis. Recently, shorter treatment, consisting of 5 consecutive doses, has presented the same efficacy results and better tolerance for the patient as a result of a quicker return to normal life [6].
Owing to increasing knowledge of the molecular and genetic profile in breast cancer and the deployment of available techniques, nowadays therapies can be more effectively personalized by adapting doses and volumes to be administered but at the same time increasing the complexity in management and prescriptions. In order to diminish local relapse as much as possible, additional doses to the tumor bed are needed with high precision in treatment administration to the tissue at risk.
2. Bed Boost in Breast Cancer
The tumor bed is the main site of relapse after conservative surgery. Pathological analysis of surgical specimens has demonstrated that the remaining tumor cells are localized 15–20 mm around the resection cavity [7]. Several factors are predictive for ipsilateral breast recurrence like age, higher grade, positive or close margins and the presence of intraductal carcinoma. In the 1980s, after demonstrating that WBI increases local control, several studies showed the efficacy of additional doses or a boost to the tumor bed. In the Lyon trial, patients younger than 70 years old received subsequent adjuvant WBI of 50 Gy with and without additional doses of 4 fractions of 2.5 Gy by means of an electron beam. Patients receiving a boost presented lower recurrence rates with increased incidence of telangiectasia but similar cosmesis between the groups after a follow-up of 3 years [8].
In the EORTC 22881 trial, patients with negative margins after lumpectomy were randomized to receive a sequential 16 Gy tumor bed boost versus no additional dose. Patients included in the boost group presented higher local control rates with a local recurrence of 7% at 10 years when compared to 10% in the no-boost group (p > 0.0001). All subgroups benefited from the increased dose but this was markedly higher in the younger patients. The boost was associated with increased rates of fibrosis and telangiectasia [9].
After WBI with moderate hypofractionation, a bed boost was administered sequentially but frequently at a classical fractionation of 2 Gy [10]. Some trials analyzed the use of a hypofractionated boost after WBI administering an additional 13.32 Gy in four fractions [11] or 3–6 fractions of 2.7 Gy [12] with favorable results in terms of tolerance and cosmesis. Thanks to the huge improvement in radiation techniques, a simultaneous integrated boost is being implemented in most centers while also utilizing ultra-hypofractionated schedules, allowing further shortening of adjuvant treatment, saving time and costs without compromising clinical outcomes [13]. This strategy has shown dosimetric advantages in terms of target-volume coverage and lower doses to organs at risk [14].
3. Intraoperative Radiotherapy in Conservative Breast Cancer Treatment
Intraoperative procedures have been implemented in recent decades as a way to increase better management of several cancers, minimizing systemic side effects by limiting the irradiated normal tissue volume and increasing the therapeutic index [15]. Also, as recently published, intraoperative management allows a theragnostic approach by diagnosing and treating at the same time during surgery [16]. Accelerated partial-breast irradiation (APBI) focuses radiation only to the tumor bed with a margin, as recurrences occur more frequently in this area and this allows shorter treatment durations while sparing healthy tissue. Several randomized trials comparing APBI to WBI demonstrated similar tumor control after five years in selected patients [17]. GEC-ESTRO and ASTRO have provided guidelines for the selection of treatment in patients eligible for APBI [18][19]. Many APBI techniques have been developed, including brachytherapy [20], external radiation therapy [21] and intraoperative radiotherapy (IORT) [22][23]. This last technique allows an extremely short radiation-treatment time during surgery and decreases hospital visits for adjuvant radiation therapy. Also, direct radiation of the surgical bed is administered and allows better protection of nearby organs at risk. IORT can be performed using mobile electron units (Figure 1a,b) or low-energy X-ray systems (Figure 1c,d).
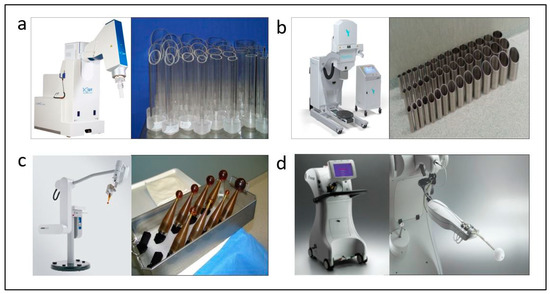
Figure 1. Intraoperative radiotherapy systems and appplicators. In the upper section electron accelerators are shown: Liac®, SIT, Vicenza, Italy (a) and Mobetron®, IntraOp, Sunnyvale, CA, USA (b), respectively. At the bottom the systems utilizing low-energy X-rays are shown: Intrabeam®, Carl Zeiss, Oberkochen, Germany (c) and XoftAxxent®, Xoft iCAd Inc., San José, CA, USA (d) and spherical applicators.
The technique of utilizing electron accelerators is one of the available options and entails, after tumor removal, remodeling the tumor bed by digitally dissecting until reaching the pectoral fascia, creating space to temporarily place a protective disk that prevents the deepening of the beam. Additionally, an approximation of the lateral margins is performed to expose the at-risk tissue for radiation. The most recent technical developments of IORT using low-energy X-ray devices offer better depth penetration and utilize spherical applicators that adapt to the tumor cavity without remodeling it, with evident advantages. These include a smaller treatment volume, a shorter learning curve and reduced radiation-protection requirements, as they do not require special isolation of the operating room. Both techniques involve only a discreet increase in operative time. See Figure 2 for comparison of positioning and dose distribution between treatment devices. Intraoperative radiotherapy has the significant advantage of greater precision in localizing the tumor cavity, immediacy of treatment that hinders tumor repopulation, and an increased immune response due to the effect of a high radiation dose at the peri-tumoral microenvironment [24]. By contrast, not all patients are suitable for the IORT procedure due to breast size or localization of the tumor in the breast. So, cautious selection of candidates to receive it is an important issue.
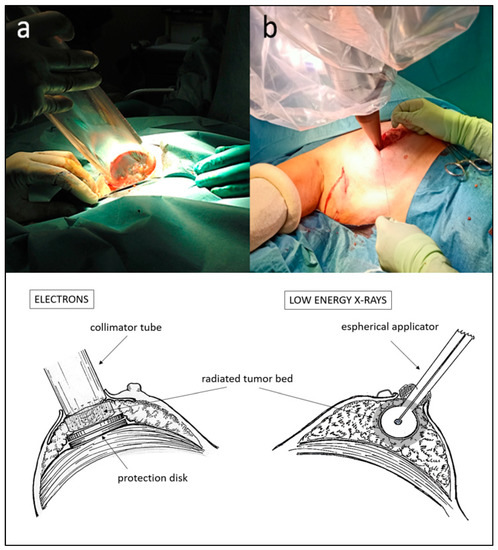
Figure 2. Positioning of IORT applicators for electron accelerators (a) and for low-energy X-rays (b) after lumpectomy. Schematic dosimetric distributions at the tumor bed in treatment position for each system are shown in the lower half of the figure.
IORT was included as an option to perform APBI, emphasizing the need for careful selection of patients to be included outside a clinical trial (“suitable group” in guidelines) [19]. For example, there are some exclusion criteria like size superior to 3 cm, extensive ductal carcinoma in situ, the presence of positive nodes or a basal-like molecular subtype, owing to an increased risk for local relapse. The first experiences of IORT were carried out with mobile accelerators that provided radiation by means of an electron beam. The ELIOT trial included 1305 patients, comparing WBI to APBI with electron IORT. At a twelve-year follow-up, the relapse rate was significantly higher in the IORT group than in the WBI group, and overall survival was not different between the two groups [25][26] (Table 1). The improved patient selection demonstrated better results in terms of disease control [27].
More recently, the TARGIT-A trial, using 50 kV X-rays with Intrabeam®, 3451 patients were randomized to receive WBI (1730 patients) or IORT (1721 patients). Wound-related complications were similar between groups, but there was significantly less grade 3 or 4 toxicity related to radiotherapy complications with IORT than with WBI [28]. Also, better cosmesis results were observed through a computer-assisted objective system [29], and overall a better quality of life [30]. With a longer follow-up (median 8.6 years, maximum 18.9 years), no statistically significant differences were found for local-recurrence-free survival (hazard ratio 1.13; 95% CI: 0.91 to 1.41; p = 0.28) [31], but there were significantly fewer deaths in the IORT group than in the WBI group (see Table 1), attributable to inferior mortality from cardiovascular causes and other cancers (45 vs. 74 events, HR 0.59, p = 0.005) This pragmatic trial also included patients that after surgery, and according to pathologic findings, needed complementary WBI. So, in approximately 25% of cases, IORT was administered as a boost and for this reason this strategy began to be utilized. Recently, the Spanish breast radiotherapy research group (GEORM) has published a national consensus on the use of IORT both for APBI and as a boost [32].
Table 1. Randomized studies evaluating IORT as partial-breast irradiation after breast-conservation surgery.
| Study | No. Patients | IORT Technique | IORT Dose (Single Fraction, Gy) | Median Follow-Up (Years) | Rate of Local Recurrence with IORT (%) | Rate of Local Recurrence with WBI (%) | Hazard Ratio (95% CI, p Value) |
|---|---|---|---|---|---|---|---|
| ELIOT [26] | 1305 | Electrons | 21 | 12.4 | 11 | 2 | 4.62 (2.68–7.95, p < 0.0001) |
| TARGIT-A [31] | 3451 | 50 kV X-rays | 20 | 8.6 | 2.11 | 0.95 | 1.13 (0.91–1.41, p = 0.28) |
This entry is adapted from the peer-reviewed paper 10.3390/cancers15164025
References
- Veronesi, U.; Marubini, E.; Mariani, L.; Galimberti, V.; Luini, A.; Veronesi, P.; Salvadori, B.; Zucali, R. Radiotherapy after breast -conserving surgery in small breast carcinoma: Long-term results of a randomized trial. Ann. Oncol. 2001, 12, 997–1003.
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 16, 1233–1241.
- Almahariq, M.F.; Quinn, T.J.; Siddiqui, Z.; Jawad, M.S.; Chen, P.Y.; Gustafson, G.S.; Dilworth, J.T. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother. Oncol. 2020, 142, 186–194.
- Whelan, T.; MacKenzie, R.; Julian, J.; Levine, M.; Shelley, W.; Grimard, L.; Lada, B.; Lukka, H.; Perera, F.; Fyles, A.; et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J. Natl. Cancer Inst. 2002, 94, 1143–1150.
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.A.; Barrett, J.M.; Barrett-Lee, P.J.; Bentzen, S.M.; Bliss, J.M.; Brown, J.; Dewar, J.A.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008, 371, 1098–1107.
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626.
- Holland, R.; Veling, S.H.; Mravunac, M.; Hendriks, J.H. Histologic multifocality of Tis, T1-2 breast carcinomas: Implications for clinical trials of breast-conserving surgery. Cancer 1985, 56, 979–990.
- Romestaing, P.; Lehingue, Y.; Carrie, C.; Coquard, R.; Montbarbon, X.; Ardiet, J.M.; Mamelle, N.; Gérard, J.P. Role of 10-Gy boost in the conservative treatment of early breast cancer. Results of a randomizerd clinical trial in Lyon, France. J. Clin. Oncol. 1997, 15, 963–968.
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhuis, C.; Horiot, J.-C.; et al. European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015, 16, 47–56.
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094.
- Gupta, A.; Khan, A.J.; Yegya-Raman, N.; Sayan, M.; Ahlawat, S.; Ohri, N.; Goyal, S.; Moore, D.F.; Eladoumikdachi, F.; Toppmeyer, D.; et al. 5-year results of a prospective phase 2 trial evaluating 3-week hypofractionated whole breast radiation therapy inclusive of a sequential boost. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 267–274.
- Sanz, J.; Rodríguez, N.; Foro, P.; Dengra, J.; Reig, A.; Pérez, P.; Membrive, I.; Ortiz, A.; Codinach, M.; Algara, M. Hypofractionated boost after whole breast irradiation in breast carcinoma: Chronic toxicity results and cosmesis. Clin. Transl. Oncol. 2017, 19, 464–469.
- Forster, T.; Köhler, C.; Dorn, M.; Häfner, M.F.; Arians, N.; König, L.; Ben Harrabi, S.; Schlampp, I.; Weykamp, F.; Meixner, E.; et al. Noninferiority of Local Control and Comparable Toxicity of Intensity Modulated Radiation Therapy With Simultaneous Integrated Boost in Breast Cancer: 5-Year Results of the IMRT-MC2 Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023. in press. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0360301623005230 (accessed on 20 June 2023).
- Franco, P.; Cante, D.; Sciacero, P.; Girelli, G.; La Porta, M.R.; Ricardi, U. Tumor bed boost integration during whole breast radiotherapy: A review of the current evidence. Breast Care 2015, 10, 44–49.
- Paunesku, T.; Woloschak, G.E. Future Directions of Intraoperative Radiation Therapy: A Brief Review. Front. Oncol. 2017, 7, 300.
- Ling, J.; Luo, Y.; Sun, C.; Dong, Z.; Wu, R.; Tang, X.; Du, N.; Zhu, R.; Chen, S.; Liu, M.; et al. Live intraoperative diagnostis of hepatic metastasis via HDACs targeting molecular theranostic agent. Chem. Eng. J. 2021, 406, 126900.
- Livi, L.; Meattini, I.; Marrazzo, L.; Simontacchi, G.; Pallotta, S.; Saieva, C.; Paiar, F.; Scotti, V.; Cardillo, C.D.L.; Bastiani, P.; et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur. J. Cancer 2015, 51, 451–463.
- Polgár, C.; Van Limbergen, E.; Pötter, R.; Kovács, G.; Polo, A.; Lyczek, J.; Hildebrandt, G.; Niehoff, P.; Guinot, J.L.; Guedea, F.; et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast conserving surgery: Recommendations of the Groupe European de Curie therapy-European Society for Therapy Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 2010, 94, 264–273.
- Correa, C.; Harris, E.E.; Leonardi, M.C.; Smith, B.D.; Taghian, A.G.; Thompson, A.M.; White, J.; Harris, J.R. Accelerated partial breast irradiation: Executive summary for the update of ASTRO evidence-based consensus statement. Pract. Radiat. Oncol. 2017, 7, 73–79.
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238.
- Mózsa, E.; Mészáros, N.; Major, T.; Fröhlich, G.; Stelczer, G.; Sulyok, Z.; Fodor, J.; Polgár, C. Accelerated partial breast irradiation with external beam three-dimensional conformal radiotherapy. Five-year results of a prospective phase II clinical study. Strahlenther. Onkol. 2014, 190, 444–450.
- Holmes, D.R. Intraoperative radiotherapy in breast conserving surgery. J. Surg. Oncol. 2014, 110, 68–74.
- Veronesi, U.; Orecchia, R.; Luini, A.; Galimberti, V.; Gatti, G.; Intra, M.; Veronesi, P.; Leonardi, M.C.; Ciocca, M.; Lazzari, R.; et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery: Experience with 590 cases. Ann. Surg. 2005, 242, 101–106.
- Massarut, S.; Belletti, B.; Segatto, I.; Piccoli, E.; Baldassarre, G. Wound response after intraoperative radiotherapy. Transl. Cancer Res. 2015, 4, 161–172.
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 2013, 14, 1269–1277.
- Orecchia, R.; Veronesi, U.; Maisonneuve, P.; Galimberti, V.E.; Lazzari, R.; Veronesi, P.; Jereczek-Fossa, B.A.; Cattani, F.; Sangalli, C.; Luini, A.; et al. Intraoperative irradiation for early breast cancer (ELIOT): Long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021, 22, 597–608.
- Veronesi, U.; Orecchia, R.; Luini, A.; Galimberti, V.; Zurrida, S.; Intra, M.; Veronesi, P.; Arnone, P.; Leonardi, M.C.; Ciocca, M.; et al. Intraoperative radiotherapy during breast conserving surgery: A study on 1,822 cases treated with electrons. Breast Cancer Res. Treat. 2010, 124, 141–151.
- Vaidya, J.S.; Wenz, F.; Bulsara, M.; Tobias, J.S.; Joseph, D.J.; Keshtgar, M.; Flyger, H.L.; Massarut, S.; Alvarado, M.; Saunders, C.; et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014, 383, 603–613.
- Corica, T.; Nowak, A.K.; Saunders, C.M.; Bulsara, M.K.; Taylor, M.; Williams, N.R.; Keshtgar, M.; Joseph, D.J.; Vaidya, J.S. Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-A Trial. Radiat. Oncol. 2018, 13, 68–77.
- Welzel, G.; Boch, A.; Sperk, E.; Hofmann, F.; Kraus-Tiefenbacher, U.; Gerhardt, A.; Suetterlin, M.; Wenz, F. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: Results from the randomized phase III trial TARGIT-A. Radiat. Oncol. 2013, 8, 9.
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ 2020, 370, m2836.
- Eraso, A.; Sanz, J.; Ibáñez, R.; Alonso, L.M.; Calin, A.; Casamayor, M.C.; Pla, M.J.; Piñero, A.; Ripoll, F.; Algara, M. Primer consenso español sobre el uso de la radioterapia intraoperatoria en el cáncer de mama. Conclusiones del panel de expertos. Rev. Senol. Patol. Mamar. 2023, 36, 100502.
This entry is offline, you can click here to edit this entry!
