Vascular endothelial growth factor (VEGF) is a major angiogenic molecule that induces choroid neovascularization (CNV). VEGF has five ligand member in human: VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor, and there are three receptors: VEGFR1, VEGFR2 and VEGFR3. VEGFs play an important role in vascular development and choroid maintenance in the normal eye. The basolateral secretion of VEGF from the retinal pigment epithelium (RPE) continues throughout life and mediates RPE survival. However, the increase in VEGF secretion from RPE and the loss of RPE polarity are causes of the pathologic CNV condition. Since the off- label bevacizumab started to be used to treat CNV, neovascular age-related macular degeneration (nAMD), there are several anti-VEGF agents approved: pegaptanib, ranibizumab, aflibercept, conbercept, brolucizumab, faricimab.
- retina
- neovascularization
- choroid
- retinal pigment epithelium
1. Bevacizumab (Avastin, Genentech)
2. Pegaptanib (Macugen, Bausch & Lomb)
3. Ranibizumab (Lucentis, Genentech)
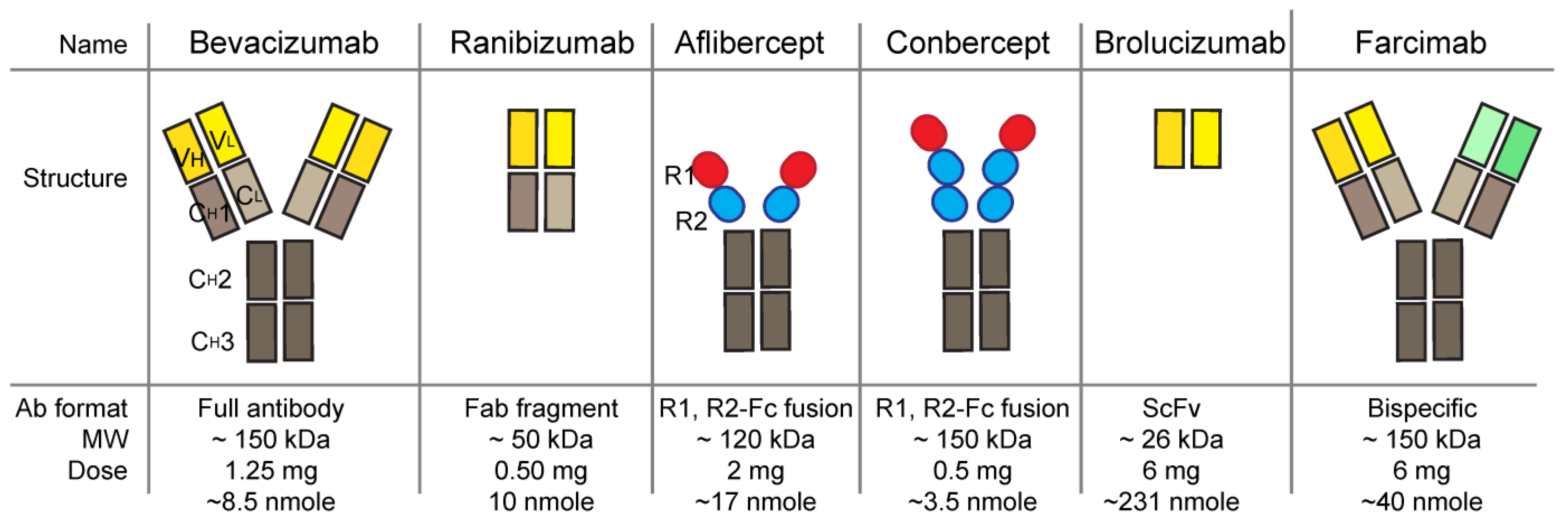
4. Aflibercept (Eylea, Regeneron Pharmaceuticals)
5. Brolucizumab (RTH258, Beovu, Novartis)
6. Conbercept (KH902, Lumitin, Chengdu Kanghong Biotechnology)
7. Faricimab (Vabysmo, Genentech)
8. Ranibizumab Ocular Implant (Susvimo, Genentech)
This entry is adapted from the peer-reviewed paper 10.3390/ijms241613004
References
- Joshua D. Stein; Paula Anne Newman-Casey; Tavag Mrinalini; Paul P. Lee; David W. Hutton; Cost-Effectiveness of Bevacizumab and Ranibizumab for Newly Diagnosed Neovascular Macular Degeneration. Ophthalmol. 2014, 121, 936-945, .
- Monika Kapur; Suvansh Nirula; Mayuresh P. Naik; Future of anti-VEGF: biosimilars and biobetters. Int. J. Retin. Vitr. 2022, 8, 1-8, .
- Sivaprasad, S. Role of pegaptanib sodium in the treatment of neovascular age-related macular degeneration. Clin. Ophthalmol. 2008, 2, 339–346. https://doi.org/10.2147/opth.s2617
- Magnussen, A.L.; Rennel, E.S.; Hua, J.; Bevan, H.S.; Beazley Long, N.; Lehrling, C.; Gammons, M.; Floege, J.; Harper, S.J.; Agostini, H.T.; et al. VEGF-A165b is cytoprotective and antiangiogenic in the retina. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4273–4281. https://doi.org/10.1167/iovs.09-4296.
- Chen, Y.; Wiesmann, C.; Fuh, G.; Li, B.; Christinger, H.W.; McKay, P.; de Vos, A.M.; Lowman, H.B. Selection and analysis of an optimized anti-VEGF antibody: Crystal structure of an affinity-matured Fab in complex with antigen. J. Mol. Biol. 1999, 293, 865–881. https://doi.org/10.1006/jmbi.1999.3192.
- Group, C.R.; Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. https://doi.org/10.1056/NEJMoa1102673
- Kapur, M.; Nirula, S.; Naik, M.P. Future of anti-VEGF: Biosimilars and biobetters. Int. J. Retin. Vitr. 2022, 8, 2. https://doi.org/10.1186/s40942-021-00343-3
- Holz, F.G.; Oleksy, P.; Ricci, F.; Kaiser, P.K.; Kiefer, J.; Schmitz-Valckenberg, S.; Group, C.-A.S. Efficacy and Safety of Biosimilar FYB201 Compared with Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 54–63. https://doi.org/10.1016/j.ophtha.2021.04.031.
- Yoon, C.K.; Oh, J.; Bae, K.; Park, U.C.; Yu, K.S.; Yu, H.G. Efficacy and safety of a new ranibizumab biosimilar CKD-701 using a pro re nata treatment regimen in neovascular age-related macular degeneration: A phase 3 randomized clinical trial. PLoS ONE 2022, 17, e0275611. https://doi.org/10.1371/journal.pone.0275611
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. https://doi.org/10.1016/j.ophtha.2012.09.006
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. https://doi.org/10.1007/s10456-011-9249-6
- Stahl, A.; Sukgen, E.A.; Wu, W.C.; Lepore, D.; Nakanishi, H.; Mazela, J.; Moshfeghi, D.M.; Vitti, R.; Athanikar, A.; Chu, K.; et al. Effect of Intravitreal Aflibercept vs Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity: The FIREFLEYE Randomized Clinical Trial. JAMA 2022, 328, 348–359. https://doi.org/10.1001/jama.2022.10564
- Tadayoni, R.; Sararols, L.; Weissgerber, G.; Verma, R.; Clemens, A.; Holz, F.G. Brolucizumab: A Newly Developed Anti-VEGF Molecule for the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmologica 2021, 244, 93–101. https://doi.org/10.1159/000513048
- Dugel, P.U.; Jaffe, G.J.; Sallstig, P.; Warburton, J.; Weichselberger, A.; Wieland, M.; Singerman, L. Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology 2017, 124, 1296–1304. https://doi.org/10.1016/j.ophtha.2017.03.057
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. https://doi.org/10.1016/j.ophtha.2019.04.017.
- Dugel, P.U.; Singh, R.P.; Koh, A.; Ogura, Y.; Weissgerber, G.; Gedif, K.; Jaffe, G.J.; Tadayoni, R.; Schmidt-Erfurth, U.; Holz, F.G. HAWK and HARRIER: Ninety-Six-Week Outcomes from the Phase 3 Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 128, 89–99. https://doi.org/10.1016/j.ophtha.2020.06.028
- Motevasseli, T.; Mohammadi, S.; Abdi, F.; Freeman, W.R. Side Effects of Brolucizumab. J. Ophthalmic. Vis. Res. 2021, 16, 670–675. https://doi.org/10.18502/jovr.v16i4.9757.
- Khanani, A.M.; Brown, D.M.; Jaffe, G.J.; Wykoff, C.C.; Adiguzel, E.; Wong, R.; Meng, X.; Heier, J.S.; Investigators, M. MERLIN: Phase 3a, Multicenter, Randomized, Double-Masked Trial of Brolucizumab in Participants with Neovascular Age-Related Macular Degeneration and Persistent Retinal Fluid. Ophthalmology 2022, 129, 974–985. https://doi.org/10.1016/j.ophtha.2022.04.028
- Karle, A.C.; Wrobel, M.B.; Koepke, S.; Gutknecht, M.; Gottlieb, S.; Christen, B.; Rubic-Schneider, T.; Pruimboom-Brees, I.; Leber, X.C.; Scharenberg, M.; et al. Anti-brolucizumab immune response as one prerequisite for rare retinal vasculitis/retinal vascular occlusion adverse events. Sci. Transl. Med. 2023, 15, eabq5241. https://doi.org/10.1126/scitranslmed.abq5241
- Lu, X.; Sun, X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des. Devel. Ther. 2015, 9, 2311–2320. https://doi.org/10.2147/DDDT.S67536
- Zhang, M.; Yu, D.; Yang, C.; Xia, Q.; Li, W.; Liu, B.; Li, H. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm. Res. 2009, 26, 204–210. https://doi.org/10.1007/s11095-008-9718-9
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. https://doi.org/10.1007/s10456-011-9249-6.
- Li, H.; Lei, N.; Zhang, M.; Li, Y.; Xiao, H.; Hao, X. Pharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbit. Exp. Eye Res. 2012, 97, 154–159. https://doi.org/10.1016/j.exer.2011.09.002.
- Stewart, M.W.; Rosenfeld, P.J. Predicted biological activity of intravitreal VEGF Trap. Br. J. Ophthalmol. 2008, 92, 667–668. https://doi.org/10.1136/bjo.2007.134874
- Cui, C.; Lu, H. Clinical observations on the use of new anti-VEGF drug, conbercept, in age-related macular degeneration therapy: A meta-analysis. Clin. Interv. Aging 2018, 13, 51–62. https://doi.org/10.2147/CIA.S151225.
- Zhou, P.; Zheng, S.; Wang, E.; Men, P.; Zhai, S. Conbercept for Treatment of Neovascular Age-Related Macular Degeneration and Visual Impairment due to Diabetic Macular Edema or Pathologic Myopia Choroidal Neovascularization: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 696201. https://doi.org/10.3389/fphar.2021.696201.
- Khanani, A.M.; Patel, S.S.; Ferrone, P.J.; Osborne, A.; Sahni, J.; Grzeschik, S.; Basu, K.; Ehrlich, J.S.; Haskova, Z.; Dugel, P.U. Efficacy of Every Four Monthly and Quarterly Dosing of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The STAIRWAY Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 964–972. https://doi.org/10.1001/jamaophthalmol.2020.2699.
- Sahni, J.; Dugel, P.U.; Patel, S.S.; Chittum, M.E.; Berger, B.; Del Valle Rubido, M.; Sadikhov, S.; Szczesny, P.; Schwab, D.; Nogoceke, E.; et al. Safety and Efficacy of Different Doses and Regimens of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The AVENUE Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 955–963. https://doi.org/10.1001/jamaophthalmol.2020.2685
- Khanani, A.M.; Guymer, R.H.; Basu, K.; Boston, H.; Heier, J.S.; Korobelnik, J.F.; Kotecha, A.; Lin, H.; Silverman, D.; Swaminathan, B.; et al. TENAYA and LUCERNE: Rationale and Design for the Phase 3 Clinical Trials of Faricimab for Neovascular Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100076. https://doi.org/10.1016/j.xops.2021.100076.
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. https://doi.org/10.1016/S0140-6736(22)00010-1.
- Nair, A.A.; Finn, A.P.; Sternberg, P., Jr. Spotlight on Faricimab in the Treatment of Wet Age-Related Macular Degeneration: Design, Development and Place in Therapy. Drug Des. Devel. Ther. 2022, 16, 3395–3400. https://doi.org/10.2147/DDDT.S368963
- Watanabe, D.; Suzuma, K.; Suzuma, I.; Ohashi, H.; Ojima, T.; Kurimoto, M.; Murakami, T.; Kimura, T.; Takagi, H. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2005, 139, 476–481. https://doi.org/10.1016/j.ajo.2004.10.004.
- Loukovaara, S.; Robciuc, A.; Holopainen, J.M.; Lehti, K.; Pessi, T.; Liinamaa, J.; Kukkonen, K.T.; Jauhiainen, M.; Koli, K.; Keski-Oja, J.; et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013, 91, 531–539. https://doi.org/10.1111/j.1755-3768.2012.02473.x.
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. https://doi.org/10.15252/emmm.201505889
- Oshima, Y.; Deering, T.; Oshima, S.; Nambu, H.; Reddy, P.S.; Kaleko, M.; Connelly, S.; Hackett, S.F.; Campochiaro, P.A. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J. Cell. Physiol. 2004, 199, 412–417. https://doi.org/10.1002/jcp.10442
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. https://doi.org/10.1016/j.ophtha.2019.03.036.
- Khanani, A.M., et al., End-of-Study Results for the Ladder Phase 2 Trial of the Port Delivery System with Ranibizumab for Neo-vascular Age-Related Macular Degeneration. Ophthalmol Retina, 2021. 5(8): p. 775-787. https://doi.org/10.1016/j.oret.2020.11.004.
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307. https://doi.org/10.1016/j.ophtha.2021.09.016.
