Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Outer membrane vesicles (OMVs) are spheroidal proteoliposomes ranging from ~20 to 200 nm in diameter that originate from the outer membrane of Gram-negative bacteria. The biogenesis of OMVs has been a topic of intense research due to the diverse roles that OMVs play in bacterial pathogenesis, immune modulation, and potential therapeutic applications.
- outer membrane vesicles (OMVs)
- DNA methylation (DNAm)
- immune modulation
1. Introduction
Gram-negative bacteria employ diverse mechanisms to interact with other bacteria and their human hosts. Among these mechanisms, outer membrane vesicles (OMVs) are critical in facilitating host–pathogen interactions without requiring direct cell-to-cell contact. This is particularly relevant when bacteria colonize the mucus that overlays host epithelial cells [1][2]. OMVs are spheroidal proteoliposomes ranging from ~20 to 200 nm in diameter that originate from the outer membrane of Gram-negative bacteria [3][4][5][6]. These vesicles contain various cytoplasmic and periplasmic components, including proteins, DNA, RNA, and metabolites [7][8][9][10]. The lipid bilayer of OMVs protects the contents from extra-vesicular proteins, such as proteases and RNases [2]. OMVs were first characterized in 1967 by Chatterjee and Das by transmission-electron microscopy of Vibrio cholerae [11]. Studies in the years since have shown that OMVs are produced by a diverse range of Gram-negative bacteria during infection and have been isolated from both pathogenic and commensal bacteria colonizing the human gut and lung. Although Gram-negative bacteria also release outer-inner membrane vesicles (O-IMVs) that derive from the bacterium’s inner and outer membrane, these vesicles account for less than 1% of the total secreted vesicles [12]. Both OMVs and O-IMVs encapsulate bacterial factors that modulate the host’s immune response to infection. These factors include proteins that inhibit epithelial chloride ion secretion and small interfering RNA (sRNA) that bind to and silence host mRNA transcripts. Despite the significant progress that has been made in characterizing the immunomodulatory properties of OMVs, many of their mechanisms of action remain elusive. Therefore, investigating the interplay between host and OMVs is crucial for developing novel therapies against bacterial infections and the ensuing inflammatory response. Recent reviews explore the intricate interactions between OMVs and the host immune response [13][14][15][16][17][18][19][20][21].
2. OMV Biogenesis
The biogenesis of OMVs has been a topic of intense research in recent years due to the diverse roles that OMVs play in bacterial pathogenesis, immune modulation, and potential therapeutic applications. The explosive cell lysis and budding models are two proposed mechanisms for OMV generation in Gram-negative bacteria (Figure 1).
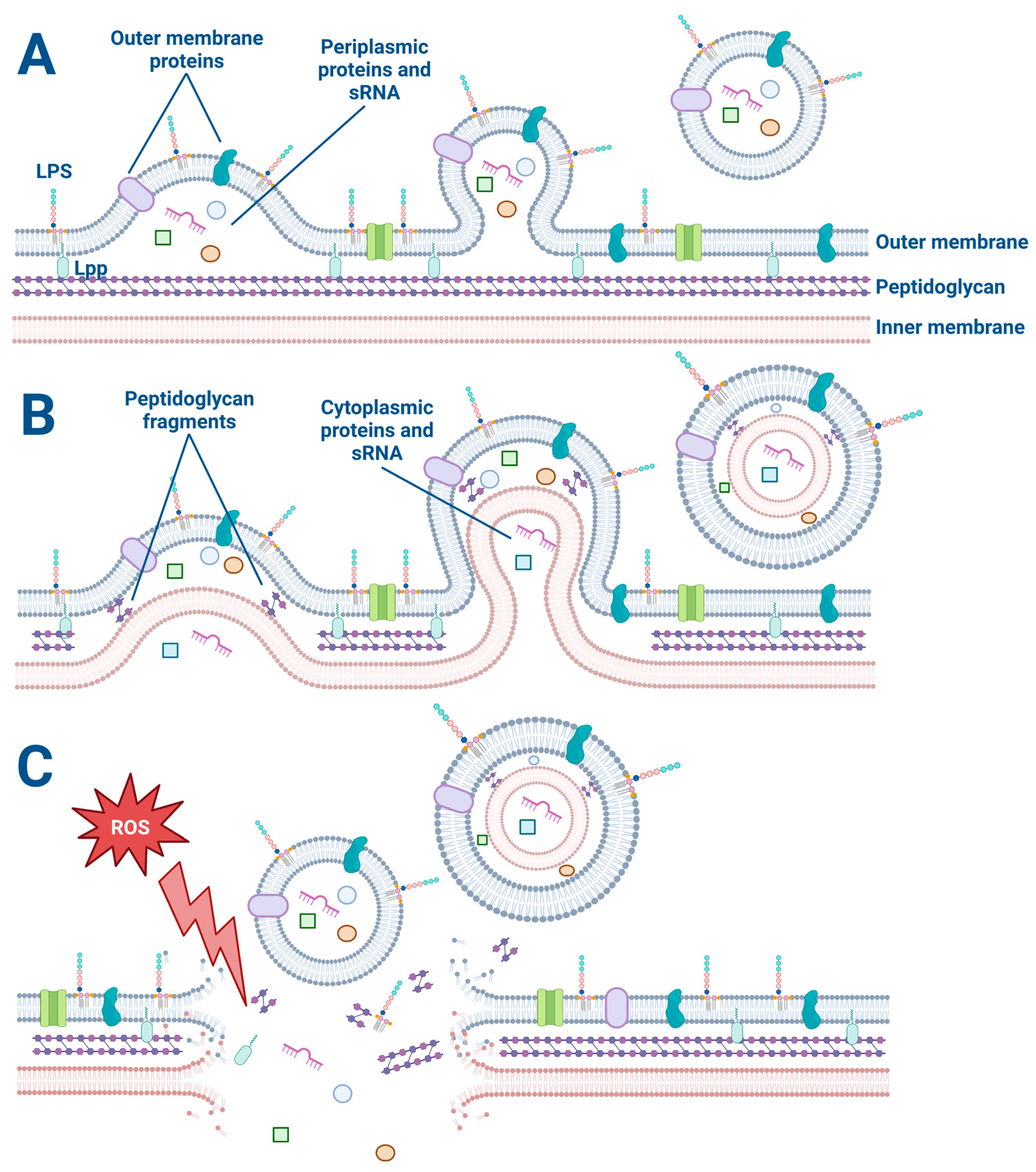
Figure 1. The explosive cell lysis and budding models of OMV and O-IMV generation. (A) The budding mechanism of OMV generation. Lipoproteins (Lpp) anchor the outer membrane to the peptidoglycan. Membrane proteins and lipopolysaccharides (LPS) decorate the outer membrane and are incorporated into the budding OMV along with periplasmic proteins and sRNA. (B) The budding model of O-IMV generation. Both cytoplasmic and periplasmic proteins and sRNA are incorporated into budding O-IMVs. (C) Cellular stress such as exposure to reactive oxygen species disrupts the membrane of a Gram-negative bacterium, causing membrane fragments to encapsulate free periplasmic and cytoplasmic material and form OMVs and O-IMVs.
The explosive cell lysis model proposes that OMVs are generated as a result of a sudden and catastrophic rupture of the bacterial cell membrane. In this model, a high amount of stress on the bacterial cell can lead to a breakdown in membrane integrity and a subsequent release of large amounts of cytoplasmic and periplasmic contents, including membrane fragments [22][23]. The released membrane fragments can spontaneously assemble into OMVs. This model is supported by observations of high levels of OMVs in bacterial cultures undergoing stress or lysis, such as during antibiotic treatment or exposure to detergents [22][23][24]. The use of a live–dead staining assay on bacteria is beneficial for all OMV studies to determine if some OMVs are formed from explosive cell lysis versus another budding mechanism [25][26]. For example, preliminary studies have revealed that low concentrations (1 μg/mL) of the antibiotic Tobramycin kill about 10% of P. aeruginosa (PA14) as determined by the live–dead assay.
On the other hand, the budding model proposes that OMVs are generated by a more controlled process involving the gradual formation and release of vesicles from the bacterial outer membrane. In this model, the budding of OMVs is thought to involve a selective packaging of cargo, such as proteins, lipids, and nucleic acids, into the vesicles [27]. Once the cargo is packaged, the OMVs are released from the outer membrane [27]. This model is supported by observations of asymmetrically shaped OMVs that display a more uniform size and cargo composition than those generated by explosive cell lysis [28]. Furthermore, OMV budding has been observed by electron microscopy of P. aeruginosa biofilms (Figure 2). In some cases, the budding of OMVs may also be triggered by bacterial stressors such as oxidative stress or exposure to antimicrobial agents [24][27].
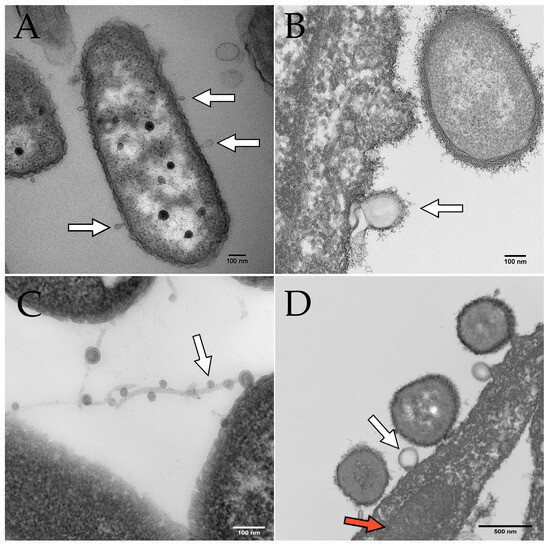
Figure 2. Electron microscopy images of OMVs budding from P. aeruginosa. Scale bars indicate 100 nm in panels (A–C) and 500 nm in panel (D). White arrows indicate OMVs in all panels. (A) OMVs budding from P. aeruginosa PAO1 cultured on human bronchial epithelial cells. (B) OMV budding from P. aeruginosa PA14 grown in Minimal Essential Medium (MEM) with 0.4% arginine. (C) OMVs on filamentous structures produced by P. aeruginosa. (D) P. aeruginosa OMV (derived from PAO1 grown in MEM with 10 mM glucose and 8 µM FeCl3) fusing with a eukaryotic cell. The red arrow indicates a mitochondrion in the airway epithelial cell.
While both the explosive cell lysis and budding models have their merits and are not mutually exclusive, it is important to note that the relative contribution of each process to OMV biogenesis may depend on the bacterial species and environmental conditions. Some studies have suggested that most OMVs are generated by the budding model [27][28][29], while others have proposed that both mechanisms can contribute equally to OMV biogenesis [30].
Further research is needed to fully understand the mechanisms involved in OMV biogenesis, how RNA, DNA, and other virulence factors are differentially packaged in OMVs, and to develop strategies for engineering OMVs with specific cargo and properties for various applications, including the development of novel treatments against chronic bacterial infections.
This entry is adapted from the peer-reviewed paper 10.3390/membranes13090752
References
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066.
- Koeppen, K.; Hampton, T.H.; Jarek, M.; Scharfe, M.; Gerber, S.A.; Mielcarz, D.W.; Demers, E.G.; Dolben, E.L.; Hammond, J.H.; Hogan, D.A.; et al. A Novel Mechanism of Host-Pathogen Interaction through SRNA in Bacterial Outer Membrane Vesicles. PLoS Pathog. 2016, 12, e1005672.
- Kikuchi, Y.; Obana, N.; Toyofuku, M.; Kodera, N.; Soma, T.; Ando, T.; Fukumori, Y.; Nomura, N.; Taoka, A. Diversity of Physical Properties of Bacterial Extracellular Membrane Vesicles Revealed through Atomic Force Microscopy Phase Imaging. Nanoscale 2020, 12, 7950–7959.
- Sheikh, A.; Zechmann, B.; Sayes, C.M.; Taube, J.H.; Greathouse, K.L. A Preparation of Bacterial Outer Membrane with Osmium Tetroxide and Uranyl Acetate Co-Stain Enables Improved Structural Determination by Transmission Electron Microscopy. Microscopy 2023, dfad027.
- Kolling, G.L.; Matthews, K.R. Export of Virulence Genes and Shiga Toxin by Membrane Vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 1999, 65, 1843–1848.
- Grenier, D.; Mayrand, D. Functional Characterization of Extracellular Vesicles Produced by Bacteroides Gingivalis. Infect. Immun. 1987, 55, 111.
- Wang, Y.F.; Fu, J. Secretory and Circulating Bacterial Small RNAs: A Mini-Review of the Literature. ExRNA 2019, 1, 14.
- Dorward, D.W.; Garon, C.F. DNA Is Packaged within Membrane-Derived Vesicles of Gram-Negative but Not Gram-Positive Bacteria. Appl. Environ. Microbiol. 1990, 56, 1960.
- Kuehn, M.J.; Kesty, N.C. Bacterial Outer Membrane Vesicles and the Host-Pathogen Interaction. Genes Dev. 2005, 19, 2645–2655.
- Diallo, I.; Provost, P. RNA-Sequencing Analyses of Small Bacterial RNAs and Their Emergence as Virulence Factors in Host-Pathogen Interactions. Int. J. Mol. Sci. 2020, 21, 1627.
- Chatterjee, S.N.; Das, J. Electron Microscopic Observations on the Excretion of Cell-Wall Material by Vibrio Cholerae. J. Gen. Microbiol. 1967, 49, 1–11.
- Pérez-Cruz, C.; Delgado, L.; López-Iglesias, C.; Mercade, E. Outer-Inner Membrane Vesicles Naturally Secreted by Gram-Negative Pathogenic Bacteria. PLoS ONE 2015, 10, e0116896.
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune Modulation by Bacterial Outer Membrane Vesicles. Nat. Rev. Immunol. 2015, 15, 375–387.
- Anand, D.; Chaudhuri, A. Bacterial Outer Membrane Vesicles: New Insights and Applications. Mol. Membr. Biol. 2016, 33, 125–137.
- Ahmed, A.A.Q.; Besio, R.; Xiao, L.; Forlino, A. Outer Membrane Vesicles (OMVs) as Biomedical Tools and Their Relevance as Immune-Modulating Agents against H. Pylori Infections: Current Status and Future Prospects. Int. J. Mol. Sci. 2023, 24, 8542.
- Chen, S.; Lei, Q.; Zou, X.; Ma, D. The Role and Mechanisms of Gram-Negative Bacterial Outer Membrane Vesicles in Inflammatory Diseases. Front. Immunol. 2023, 14, 1157813.
- Tiku, V.; Tan, M.W. Host Immunity and Cellular Responses to Bacterial Outer Membrane Vesicles. Trends Immunol. 2021, 42, 1024–1036.
- Kim, J.Y.; Suh, J.W.; Kang, J.S.; Kim, S.B.; Yoon, Y.K.; Sohn, J.W. Gram-Negative Bacteria’s Outer Membrane Vesicles. Infect. Chemother. 2023, 55, 1.
- Zhao, G.; Jones, M.K. Role of Bacterial Extracellular Vesicles in Manipulating Infection. Infect. Immun. 2023, 91, e0043922.
- Stanton, B.A. Extracellular Vesicles and Host–Pathogen Interactions: A Review of Inter-Kingdom Signaling by Small Noncoding RNA. Genes 2021, 12, 1010.
- Gilmore, W.J.; Johnston, E.L.; Zavan, L.; Bitto, N.J.; Kaparakis-Liaskos, M. Immunomodulatory Roles and Novel Applications of Bacterial Membrane Vesicles. Mol. Immunol. 2021, 134, 72–85.
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive Cell Lysis as a Mechanism for the Biogenesis of Bacterial Membrane Vesicles and Biofilms. Nat. Commun. 2016, 7, 11220.
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163.
- McBroom, A.J.; Kuehn, M.J. Release of Outer Membrane Vesicles by Gram-Negative Bacteria Is a Novel Envelope Stress Response. Mol. Microbiol. 2007, 63, 545.
- Rodriguez, B.V.; Kuehn, M.J. Staphylococcus Aureus Secretes Immunomodulatory RNA and DNA via Membrane Vesicles. Sci. Rep. 2020, 10, 18293.
- Melo, J.; Pinto, V.; Fernandes, T.; Malheiro, A.R.; Osório, H.; Figueiredo, C.; Leite, M. Isolation Method and Characterization of Outer Membranes Vesicles of Helicobacter Pylori Grown in a Chemically Defined Medium. Front. Microbiol. 2021, 12, 1253.
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 605.
- Haurat, M.F.; Elhenawy, W.; Feldman, M.F. Prokaryotic Membrane Vesicles: New Insights on Biogenesis and Biological Roles. Biol. Chem. 2015, 396, 95–109.
- Zhou, L.; Srisatjaluk, R.; Justus, D.E.; Doyle, R.J. On the Origin of Membrane Vesicles in Gram-Negative Bacteria. FEMS Microbiol. Lett. 1998, 163, 223–228.
- Zavan, L.; Fang, H.; Johnston, E.L.; Whitchurch, C.; Greening, D.W.; Hill, A.F.; Kaparakis-Liaskos, M. The Mechanism of Pseudomonas aeruginosa Outer Membrane Vesicle Biogenesis Determines Their Protein Composition. Proteomics 2023, 23, 2200464.
This entry is offline, you can click here to edit this entry!
