Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Generally, the term ‘cannabinoids’ refers to a heterogeneous family of compounds that exhibit activity upon particular human cannabinoid receptors, namely CB1 and CB2. They encompass the natural compounds present in the Cannabis plants, lipid mediators called ECs naturally produced by human cells, as well as by all vertebrates on planet Earth, and the synthetic analogs of both groups designed by scientist, called SCs.
- bacterial resistance
- methicillin-resistant S. aureus (MRSA)
- multi drug resistant (MDR) bacteria
- Cannabis sativa
- phytocannabinoids (PCs)
1. Introduction
Given the rapid emergence of multi drug resistant (MDR), extensively drug-resistant (XDR) and pandrug-resistant (PDR) pathogens, against which current antibiotics are no longer functioning, we are rapidly moving into a post-antibiotic era where infections will be practically untreatable [1]. According to the definition of the World Health Organization (WHO), antimicrobial resistance is a natural event that occurs when microbes become tolerant to drugs originally active, thus rendering several infections more difficult or impossible to treat [2][3]. Particularly, WHO has identified twelve families of bacteria to be considered as the most dangerous to human health. These families have been assigned to three priority groups, comprising critical pathogens (Acinetobacter, Pseudomonas, and Enterobacteriaceae), high priority pathogens (Enterococcus faecium, Staphylococcus aureus, Helicobacter pylori, Campylobacter, Salmonella spp., and Neisseria gonorrhoeae), and medium priority pathogens (Streptococcus pneumoniae, and Shigella spp.) [3][4]. Resistance in bacteria can be acquired or natural, but several mechanisms exist by which pathogens can become resistant to antibiotics (Figure 1).
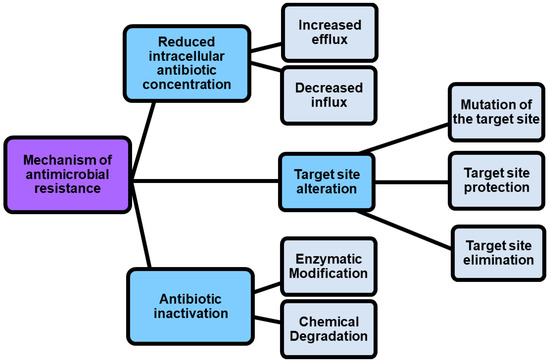
Figure 1. Mechanisms by which pathogens can become resistant.
As shown in Figure 1, antimicrobial resistance mechanisms include drug inactivation, decreased intracellular drug concentration, and altered drug targets [5].
1.1. Mechanism of Antimicrobial Resistance vs. Strategies to Develop Novel Antibiotics
Drug inactivation can occur either by enzymatic or chemical degradation, while decreased intracellular drug concentration can occur because of increasing drugs efflux and decreasing drugs influx [5]. In this regard, porin mutations in resistant strains alter the permeability of bacterial membranes, thus reducing the uptake of antibiotics into the bacterial cell. On the contrary, the hyperexpression of efflux pumps, which pump antibiotics out of the cell, dramatically reduces their concentration inside the cell [6]. Also, by the action of enzymes that chemically modify components of the bacterial outer membrane essential for antibiotic binding, some Gram-negative bacteria such as P. aeruginosa, Acinetobacter baumannii and others develop resistance to glycopeptide and polymyxin antibiotics. Furthermore, methyltransferases are a class of enzymes capable to modify the target thus promoting the resistance to antibiotics including aminoglycoside, lincosamide, macrolide, streptogramin, and oxazolidinone [7]. Another phenomenon known as “target protection” occurs when antibiotic target’s resistance proteins, such as the tetracycline ribosomal protection proteins (TRPPs), protect bacteria from the antibiotic-induced inhibition [8]. Additionally, the antibiotic resistance could be caused by the use of antibiotics in feed diet for animal production. The overuse, abuse, and misuse of β-lactams, aminoglycosides, tetracyclines, macrolides, and other antibiotics, with the purpose of promoting the development of animals, can cause the presence of residual antibiotics in the products intended for human consumption obtained from those animals, and can determine antibiotics pollution into the environment [9][10][11]. It was reported that some bacterial infections in humans are sustained by animal pathogens, namely zoonotic pathogens, thus proving that antibiotic resistance can be directly or indirectly transmitted from animal to humans [9]. A few practices, including the improvement of animal feed, waste management, and animal natural immunity, as well as the use of antibiotic alternatives such as prebiotics, probiotic vaccines, and bacteriophages can regulate and limit the antibiotic resistance, thus maintaining the potency of the available drugs [12]. However, more strategies to counteract antibiotic resistance are necessary, and currently they include the use of nanotechnology, computational methods, the use of antibiotic alternatives, drug repurposing, the synthesis of novel antibacterial agents, prodrugs, the development of efficient diagnostic agents also named rapid diagnostic tests (RDTs), the use of combination therapy, as well as the awareness, and knowledge of antibiotic prescribing (Table 1).
Table 1. Strategies for combating antibiotic resistance.
| Strategies for Combating Antibiotic Resistance | Ref. | |
|---|---|---|
| Nanotechnology | Quality by design (QbD) approach | [13] |
| Computational methods | In silico modelling | [14] |
| Fragment-based drug design (FBDD) | [15] | |
| Antibiotic alternatives | Antimicrobial peptides (AMPs) | [12] |
| Essential oils | ||
| Anti-Quorum Sensing (QS) | ||
| Darobactins | ||
| Vitamin B6 | ||
| Bacteriophages | ||
| Odilorhabdins | ||
| 18-β-glycyrrhetinic acid | ||
| Cannabinoids | ||
| Drug reproposing | Ticagrelor | [16] |
| Mitomycin C (MMC) | ||
| Auranofin | ||
| Pentamidine | ||
| Zidovudine (AZT) | ||
| Synthesis of novel antibacterial agents | Lactones | [17] |
| Piperidinol | [18] | |
| Sugar-based bactericides | [19] | |
| Isoxazole derivatives | [20] | |
| Carbazole | [21] | |
| Prodrugs | Siderophores | [22] |
| Carbapenem-oxazolidinones | ||
| Oral Gyrb/ParE dual binding inhibitor | ||
| AMPs prodrugs | ||
| Development of efficient diagnostic agents (RDTs) |
Point-of-care tests (POCTs) Molecular (genotyping) assays |
[23] |
| Combination therapy | Penicillin with streptomycin * Rifampin–isoniazid–pyrazinamide ** Trimethoprim-sulfamethoxazole Quinupristin-dalfopristin Bacitracin-polymyxin B Bacitracin-polymyxin B-gramicidin Neomycin,-bacitracin-gramicidin |
[24] |
| β-Lactams antibiotics-β-Lactamase inhibitors *** |
[25][26] | |
| Awareness and knowledge of antibiotic prescribing | [27] | |
RDTs = rapid diagnostic tests; * For enterococcal infections; ** in the treatment of tuberculosis; *** ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam.
1.2. Cannabinoids as Strategic Compounds to Develop New Antibiotics
Omitting to comment on each strategy reported in Table 1, and instead focusing on the development of alternative antibiotics, it can be observed that cannabinoids, better known for many other pharmacological and psychotropic effects are included in this category. Particularly, cannabinoids are prenylated polyketides produced in Cannabis plants and particularly in Cannabis sativa, which is an herbaceous plant that has been used for millennia for both medicinal and recreational purposes. C. sativa possesses a plethora of pharmacological properties and mind-altering effects, largely due to its content in cannabinoids, more precisely phytocannabinois (PCs), given their vegetable origin [28]. Collectively, more than 1600 chemical compounds have been isolated from C. sativa, of which over 500 are phytochemicals including cannabinoids, flavonoids terpenoids and sterols [28]. Among phytochemicals, more than 180 are cannabinoids, about 125 have been isolated, that can be classified into 11 structural families [28][29]. The most abundant representatives of these families are Δ9-tetrahydrocannabinol (Δ9-THC, also the main psychoactive cannabinoid), cannabidiol (CBD), and cannabichromene (CBC). Additionally, other classes whose prototypes are Δ8-E-tetrahydrocannabinol (Δ8-THC), cannabigerol (CBG), cannabinodiol (CBND), cannabielsoin (CBE), cannabicyclol (CBL), cannabinol (CBN), cannabitriol (CBT), and a miscellaneous group have been identified [28][29]. Currently, despite its psychotropic effects, Δ9- THC is used as therapeutic agent in the treatment of chemotherapy-associated nausea and vomiting, AIDS related loss of appetite, as well as pain and muscle spasms in multiple sclerosis [30]. Also, its carboxylic acid precursor, THCA, not exerting psycho-active effects in humans, is currently examined for its immunomodulatory, anti-inflammatory, neuroprotective and anti-neoplastic effects as well for its effectiveness in reducing adiposity and preventing metabolic disease caused by diet-induced obesity [31]. CBD, non-psychotropic as well, is currently investigated for application in the treatment of Alzheimer’s disease, Parkinson’s disease, epilepsy, cancer and for its neuroprotective efficacy [32]. Although the most studied cannabinoids for medicinal purposes are CBD and Δ9-THC, nowadays the research focus moves increasingly towards other PCs, such as the not psychoactive CBC, currently investigated for its anti-inflammatory, anti-fungal, antibiotic and analgesic effects [30], CBG and cannabigerolic acid (CBGA), which is the precursor of the decarboxylated CBG and could be considered as the “mother of all cannabinoids” (see later). Particularly, CBG has many putative benefits ranging from anti-inflammatory action to pain reliever [33]. Among other more investigated therapeutic properties, PCs including Δ9-THC, Δ8-THC, CBD, CBN, CBG, and CBC and some their correspondent carboxylic acids have shown from moderate to potent antimicrobial properties mainly against Gram-positive bacteria (MICs 0.5–8 µg/mL), and especially against strains of S. aureus, including MRSA, EMRSA, as well as fluoroquinolone and tetracycline-resistant strains, [34]. Particularly, even if the precise mechanisms used by PCs remains unknown so far, recent investigations have revealed that PCs inhibits bacteria by injuring their cytoplasmic membrane [35][36]. Recently, Luz-Veiga et al. have reported the antibacterial activity of both CBD and CBG, being CBG the most potent compound, and their capability to inhibit Staphylococci adherence to keratinocytes without compromising skin microbiota, thus being very promising as antibacterial agents to treat skin infection by topical administration [37]. Blaskovich et al., in addition to confirm the antibacterial activity of CBD on Gram-positive pathogens, including highly resistant S. aureus, S. pneumoniae, and Clostridioides difficile, demonstrated that CBD has excellent activity against biofilms, little propensity to induce resistance, and topical in vivo efficacy [38]. Moreover, the authors reported that CBD can selectively kill a subset of Gram-negative bacteria that includes the ‘urgent threat’ pathogen Neisseria gonorrhoeae [38]. Additionally, the interaction of CBD with broad-spectrum antibiotics such as ampicillin, kanamycin, and polymyxin B was studied by Gildea et al. [39]. By disrupting membrane integrity at extremely low dosages, CBD-antibiotic co-therapy showed synergistic activity against Salmonella typhimurium, offering an intriguing alternative in the treatment of this clinically relevant bacterium. The impressively strong antibacterial activity against MRSA of CBG has been reported by Farha et al. in the year 2020 [33]. Even in comparison with standard therapy with vancomycin, CBG outcompetes classical approaches against MRSA. Additionally, CBG demonstrated to inhibit the capability of MRSA to generate de novo biofilm, showed to succeed in disaggregating the pre-formed biofilm, to kill rapidly stationary phase cells (persisters), and to effectively inhibit MRSA also in vivo, in a murine model. The authors speculated that C. sativa may produce PCs as a natural defense mechanism against pathogens and suggested PCs as a new compound class serving as novel antibiotic drug [33].
Unfortunately, since in C. sativa, CBGA is promptly and directly converted to CBDA and THCA, leaving no CBGA pool available to form CBG, the CBG levels in plants are exceptionally low. In this context, it has been suggested that a possible strategy to increase the CBG yield from hemp biomass could consist in harvesting much earlier in the ripening phase of the plants before the other cannabinoids are formed and detract the CBGA from the cannabinoid pool [40]. On the other hand, having available reliable synthetic procedures to prepare natural PCs would consent the accessibility to considerable quantities of CBG, as well as of other microbiologically promising minor cannabinoids, unlikely provided naturally by Cannabis plants, thus allowing further studies finalized to the development of novel PCs-based antibiotics.
2. Phytocannabinoids (PCs), Endocannabinoids (ECs) and Synthetic Cannabinoids (SCs)
2.1. Phytocannabinoids (PCs) and Endocannabinoids (ECs)
Generally, the term ‘cannabinoids’ refers to a heterogeneous family of compounds that exhibit activity upon particular human cannabinoid receptors, namely CB1 and CB2 [41][42]. They encompass the natural compounds present in the Cannabis plants, lipid mediators called ECs naturally produced by human cells, as well as by all vertebrates on planet Earth, and the synthetic analogs of both groups designed by scientist, called SCs [42]. Natural cannabinoids from Cannabis are more specifically called PCs referring to their original plant source, differently from ECs which are produced from human cells [43][44]. PCs and ECs could include compounds structurally very different both between the two families and inside the same class, as shown in Figure 2 and Figure 3, which report the structure of the most relevant PCs and ECs, respectively.
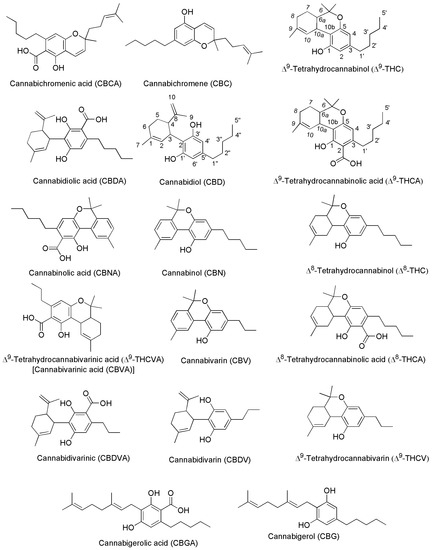
Figure 2. Chemical structure of the main PCs found in C. sativa acting on CB1 and/or CB2 receptors.
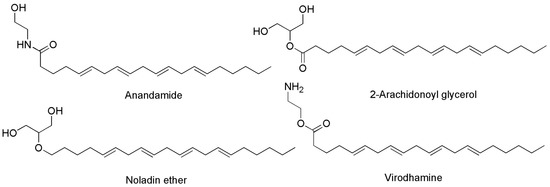
Figure 3. Chemical structure of the main ECs found in humans acting on CB1 and/or CB2 receptors.
Both PCs and ECs exert their effects by interacting with CB1 and CB2 receptors, found throughout the human body, and whose locations have been listed in Table 2. Table 2 has constructed using the valuable information contained in the relevant work by Fraguas-Sánchez et al. [45].
Table 2. Locations of CB1 and CB2 receptors in the human body.
| Receptor Type | Location | Sublocation | Ref. |
|---|---|---|---|
| CB1 | Central nervous system (CNS) | Hippocampus, cerebellum, basal ganglia, cortical regions Olfactory areas |
[46] |
| Peripheral nerve terminals Extra-neuronal sites |
Eye, vascular endothelium, adipose tissue, lungs, liver Spleen, kidneys, uterus, prostate, testis, stomach, placenta Skeletal, muscles |
||
| CB2 | Peripheral immune system tissues | Spleen, tonsils, thymus, lymph nodes | [47] |
| Peripheral immune system cells | B cells, natural killer cells, monocytes, macrophages Neutrophils, CD8+ T cells, CD4+ T cells |
||
| CNS * | Cerebellum, olfactory tubercle, striatum Thalamic nuclei (hippocampus and amygdala) |
* Under certain circumstances, most notably during inflammation.
The activation of CB1 receptor or the concurrent activation of both receptors by ECs or PCs leads to both psychotropic, undesired effects and therapeutic outcomes. Exactly, while mind alteration, psychotropic effects, cardiovascular adverse events can occur, analgesic, sedative, antidepressant, anti-inflammatory, anti-anorexic, anti-emetic, anticancer and antibacterial desirable effects can also arise. As an example, the FDA-approved drug formulations containing the synthetic versions of Δ9-THC namely dronabinol (marketed as Marinol® or Syndros®) or nabilone (marketed as Cesamet™), as well as the extracted THC (marketed as Sativex®), possess affinity for both CB1 and CB2 [48]. Clinically, they are primarily used to treat the chemotherapy-induced nausea, to enhance appetite in cachexic AIDS-patients, and to alleviate the spasticity and pain associated with multiple sclerosis [30]. Unfortunately, evidence of undesired psychotropic and cardiovascular adverse effects strongly limits the therapeutic efficacy of such medicines [49]. Otherwise, the selective activation of CB2 receptors, occurring for example by CBG or CBD, could provide therapeutic effects, such as immuno-modulatory properties, anti-inflammatory, anti-emetic, and anti-anorexic effects without exerting the psychotropic actions deriving from the CB1 activation. In addition, the potent analgesic effects, associated with the activation of CB2, could be helpful in alleviating chronic widespread musculoskeletal pain (CWP) disorders, such as fibromyalgia syndrome [50]. Also, the selective activation of CB2 receptors could enhance severe human diseases as osteoporosis, atherosclerosis, cancer, chronic liver injuries and neurodegeneration [48]. Collectively, CB1 and CB2 receptors together with ECs make part of the so-called EC system (ECS), which was discovered in the 1990′s by scientists researching cannabinoids, which includes also several enzymes involved in producing and recycling ECs. In humans, ECs are naturally produced by cells within the body in response to external factors, like pain or temperature. As shown in Figure 3, among other molecules, ECs include the well-known compounds 2-arachidonoylglycerol (2-AG) and anandamide (ANA), as well as the less-known ECs like virodhamine, and 2-arachidonoyl glycerol ether [51]. Particularly 2-AG and ANA activate both CB1 and CB2 receptors with affinity for CB1 higher than that for CB2 [48]. Collectively, the interaction between ECs and their corresponding receptors is pivotal in maintaining the body’s internal balance or homeostasis. ECs regulate some very important aspects of human health, as depicted in Figure 4.
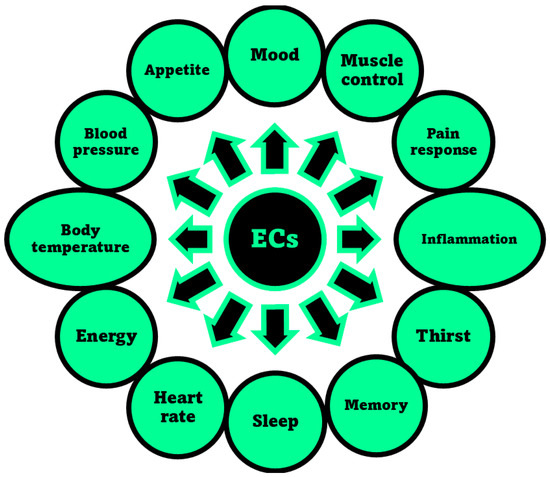
Figure 4. Aspects of humans’ life regulated by the ECS, through the interaction of ECs with receptors CB1 and/or CB2, as reported in the relevant review by Sharma et al. [52].
Researchers suggest that ECs deficiencies could cause many refractory health conditions, such as depression, arthritis, fibromyalgia, and Crohn’s disease that could ameliorate upon treatments with Cannabis, due to the activation by PCs of the same receptors activated in normal conditions by ECs. In fact, as reported above, PCs specifically produced by Cannabis plants and not by humans, when appropriately assumed can interact with CB1 and/or CB2 receptors triggering effects similar to those prompted by ECs, thus influencing the same aspects reported in Figure 4 and contributing to maintain or recover the body’s internal balance or homeostasis [41]. Anyway, if abused, the psychotropic and undesired side effects of psychoactive PCs, such as THC and CBN may overwhelm the benefits. Figure 5 shows the chemical structure of two metabolites which form in the human body after Cannabis consumption, of cannabicyclolic acid (CBLA), a degradative byproduct of cannabichromenic acid (CBCA), and of cannabicyclol (CBL) which is the product of decarboxylation of CBLA [53]. Particularly, CBL is a non-psychoactive cannabinoid, which could also derive by degradation of CBC through natural irradiation or under acid conditions [54].
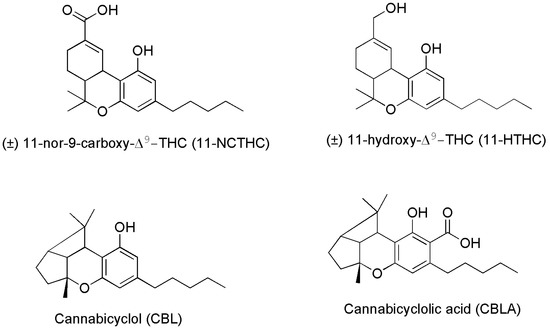
Figure 5. Chemical structure of two THC metabolites (11-NCTHC and 11-HTHC) and of two products deriving from CBCA degradation.
Particularly, CBLA, like CBCA and CBC, is a minor cannabinoid found in low concentrations in the Cannabis plant. It is not produced by Cannabis directly, but it forms when CBCA degrades after exposure to ultraviolet (UV) light or heat. CBLA is an acidic cannabinoid, like THCA, CBCA and CBDA, which produces CBL, upon decarboxylation and release of CO2. CBLA is not considered intoxicating, is often deemed non-psychoactive or non-psychotropic, and curiously, it does not interact with receptors CB1 and CB2. There is little research into the effects and potential therapeutic uses of CBLA and CBL. Anyway, although more research is needed to confirm these attributes, some suggestions exist, that CBLA may have anti-inflammatory, antimicrobial, and antitumoral effects, due to its structural similarity to CBCA and CBN [53]. As for the metabolite 11-NCTHC, it is a no longer active secondary metabolite of THC, which forms in the body through the oxidation of the still psychoactive metabolite of THC, 11-HTHC, by liver enzymes [55].
Structural Differences between Psychotropic and Not-Psychotropic PCs
It has been reported that the n-pentyl chain at the C-(3) position (Figure 2) works and essential role in the activity of psychotropic THC derivatives and that modification in this side chain leads to critical changes in the affinity, selectivity and pharmaco-potency of these ligands relating to the CB1 and CB2 cannabinoid receptors. Generally, while a shorter alkyl chain reduces the affinity of the compound for the cannabinoid receptor, an increase in the number of carbon atoms (hexyl, heptyl, or octyl) leads to an increase affinity for the same cannabinoid receptor [56][57]. Additionally, a number of other transformations in the tricyclic core of the THC cannabinoid structure have been carried out [58]. Particularly, the pyran ring-opening generally causes in the achieved compound a relative reduction in the affinity to the CB1/CB2 cannabinoid receptors, and in the psycho activity. In this regard, the absence of the tricyclic core in CBC, CBD and CBG for CB2 receptors, could be responsible for the for their higher affinity for CB2 receptors dealing with beneficial pharmacological properties, thus not exerting psychotropic effects [59].
2.2. Synthetic Cannabinoids (SCs)
The third group of cannabinoids consists of synthetic analogs of both ECs and PCs groups, appositely designed by scientists in the field, to enhance the benefits and therapeutic properties of ECs and PCs, while reducing the psychotropic and adverse effects. Among others, they include the compounds reported as examples in Figure 6 (chemical structures), and Table 3 (pharmacological properties and selectivity for receptors CB1 and CB2), which have demonstrated to be promising for treating severe humans’ chronic diseases including breast and prostate tumors, the unpleasant side-effects of chemotherapy, and chronic pain [42].
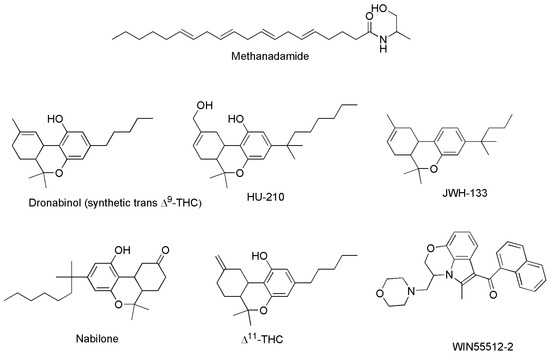
Figure 6. Structure of some SCs capable to act on CB1 and/or CB2 receptors.
Table 3. Selectivity of some SCs for CB1 and CB2 receptors, and their effects.
| SCs | Binding Affinity | Effects | Refs. | |
|---|---|---|---|---|
| CB1 (Ki, nM) | CB2 (Ki, nM) | |||
| Dronabinol * | 15 | 51 | Appetite stimulant Psychotropic effects Analgesic ↓ Nausea Antiemetic |
[60] |
| Methanandamide (AM-356) | 20 | 815 | [61] | |
| SR144528 | 280 | 0.1 | Anti-inflammatory Analgesic ↓ Neuropathic pain |
[48] |
| Cannabinor # (PRS-211,375) |
5585 | 17.4 | [62] | |
| CP47,597 | 2.1 (Kd) | 56 | Analgesic | [48] |
| JTE907 | 490 | 2.2 | Anti-inflammatory | [48] |
| JWH133 | 680 | 3.4 | ↓↓ Neurotoxicity Anti-inflammatory ↓ Alzheimer symptoms |
[48] |
| AM1241 | 272 | 3.4 | Analgesic effects ↓ Hyperalgesia ↓ Allodynia Amyotrophic lateral sclerosis |
[48] |
| GW405,833 | 8640 | 7.2 | ↓ Hyperalgesia ↓ Allodynia |
[48] |
| L-759,633 | 1604 | 9.8 | Analgesic Antianxiety Antidepressant Anti-inflammatory ↓ Alzheimer symptoms |
[48] |
| JWH139 | 2290 | 14 | ||
| HU308 | 115,000 | 23 | ||
| AM630 | 3795 | 32 | ||
| HU-210 | 0.061 | 0.52 | [63] | |
| L-759,656 | 4888 | ↑11.8 | [64] | |
| WIN 55,212-2 | 1.9 | Analgesic Anti-inflammatory ↓ Alzheimer symptoms |
[65] | |
| JWH015 | 383 | 13.8 | Analgesic Anti-inflammatory |
[66] |
| WIN 48,098 (Pravadoline) | 4.9 (IC50) | Analgesic Anti-inflammatory |
[67] | |
Ki = Defined kinetically as the ratio of rate constants koff/kon for the binding of a ligand to the receptor. This is the same as Kd; IC50 = the concentration of ligand required to saturate half of the receptor; * Approved by the FDA as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting only; # failed in Phase IIb human clinical trials due to lack of efficacy; ↓ = reduction of; ↓↓ = strong reduction of.
On the base of their affinity and selectivity for receptors CB1 and CB2, they can exert both therapeutic and psychotropic effects, or mainly one of the two. Table 3 summarizes the selectivity of some SCs for CB1 and CB2, and their therapeutic effects.
The following Figure 7 shows the chemical structures of compounds in Table 3 not previously reported in Figure 6.
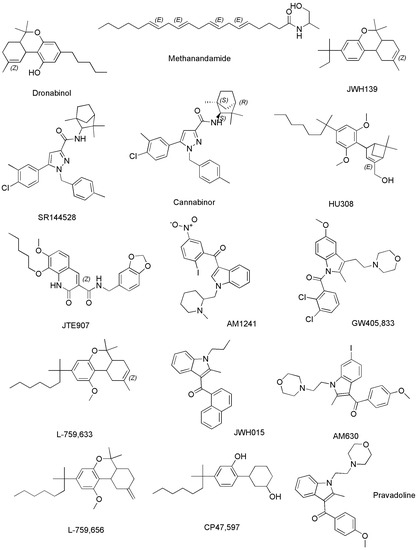
Figure 7. Structure of SCs capable to act on CB1 and/or CB2 reported in Table 3 and not previously shown in Figure 6.
Methanandamide (AM-356) is a synthetically constructed stable chiral analog of anandamide. AM-356 acts on the cannabinoid receptors, and specifically on CB1-type receptors in the CNS found in mammals, fish, and certain invertebrates (e.g., Hydra), thus resulting also a psychoactive compound [68]. HU-210, as well as other SCs including L-759,656, HU-308, L-759,633, L-768,242 etc. are potent analgesic and anti-inflammatory compounds with many of the same effects as natural THC [44]. WIN 55,212-2 is an organic heterotricyclic SC. Particularly, it is the 5-methyl-3-(morpholin-4-ylmethyl)-2,3-dihydro [1,4]oxazine [2,3,4-hi]indole substituted at position 6 by a 1-naphthylcarbonyl group. It has a role as an analgesic, and neuroprotective agent, as well as an apoptosis inhibitor [69].
JWH-133 is a Δ9-tetrahydrocannabinol lacking the hydroxy group and having a 1,1-dimethylbutyl group at position 3 in place of the pentyl group. It acts as potent and highly selective CB2 receptor agonist, thus exerting antineoplastic effects, and working as a vasodilator and an anti-inflammatory agent, as an apoptosis inhibitor, as well as an analgesic molecule [70].
Δ11-THC, also known as exo-tetrahydrocannabinol, is a synthetic isomer of tetrahydrocannabinol, developed in the 1970s. It can be synthesized from Δ8-THC by several different routes, and only the (6aR, 10aR) enantiomer is known. In animal studies in mice, it was found to exert the same effect of Δ9-THC with around 1/4 its potency. It has been identified as a component of “vaping liquids” sold for use in humans [71].
2.3. Cannabinoids Clinically Approved
Collectively, PCs and the several developed SCs have proved to be useful in the treatment of chemotherapy side effects such as nausea, vomiting, pain, weight loss, and lack of appetite [30], but only few drugs based only on THC and CBD have been approved so far in some countries, as palliative agents in anticancer treatments. Dronabinol, the synthetic analogous of Δ9-THC and nabilone, a SC similar to Δ9-THC, are currently approved in Canada, United States, and several countries in Europe to treat nausea and vomiting associated with chemotherapeutic treatments [72]. An oromucosal spray containing a mixture 1:1 of Δ9-THC and CBD marketed as Sativex® is approved in Europe and Canada for the treatment of spasticity associated with multiple sclerosis (MS), while in Canada Sativex is applied also as an adjunctive analgesic for the treatment of pain in patients with advanced cancer and MS [73][74]. An oral solution of CBD, marketed as Epidiolex® is an US FDA-approved prescription that is used in association with clobazam to treat refractory epilepsy due to Lennox–Gastaut or Dravet syndrome [75][76]. Finally, the case of Rimonabant (or SR141716) is signalized, which was marketed as Acomplia®. It is an inverse agonist for the CB1 receptor, capable to reduce the appetite, and was clinically applied as an anorectic anti-obesity drug. It was withdrawn from the market in 2009, after a long dispute between the European Medicines Agency (EMA) and the Cochrane Collaboration, because it increased the risk of psychiatric problems and suicide [77].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15071889
References
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908.
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310.
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 3 May 2023).
- Stojković, D.; Petrović, J.; Carević, T.; Soković, M.; Liaras, K. Synthetic and Semisynthetic Compounds as Antibacterials Targeting Virulence Traits in Resistant Strains: A Narrative Updated Review. Antibiotics 2023, 12, 963.
- Chancey, S.T.; Zahner, D.; Stephens, D.S. Acquired inducible antimicrobial resistance in Gram-positive bacteria. Future Microbiol. 2012, 7, 959–978.
- Spengler, G.; Kincses, A.; Gajdacs, M.; Amaral, L. New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria. Molecules 2017, 22, 468.
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782.
- Wilson, D.N.; Hauryliuk, V.; Atkinson, G.C.; O’Neill, A.J. Target protection as a key antibiotic resistance mechanism. Nat. Rev. Microbiol. 2020, 18, 637–648.
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269.
- Guetiya Wadoum, R.E.; Zambou, N.F.; Anyangwe, F.F.; Njimou, J.R.; Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A.; et al. Abusive use of antibiotics in poultry farming in Cameroon and the public health implications. Br. Poult. Sci. 2016, 57, 483–493.
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 88, 112–122.
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trend. Microbiol. 2019, 27, 323–338.
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427.
- Sarkar, D.J.; Mohanty, D.; Raut, S.S.; Das, B.K. Antibacterial properties and in silico odelling perspective of nano ZnO transported oxytetracycline-Zn2+ complex + against oxytetracycline-resistant Aeromonas hydrophila. J. Antibiot. 2022, 75, 635–649.
- Li, Q. Application of Fragment-Based Drug Discovery to Versatile Targets. Front. Mol. Biosci. 2020, 7, 180.
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief Overview of Approaches and Challenges in New Antibiotic Development: A Focus On Drug Repurposing. Front. Cell. Infect. Microbiol. 2021, 11, 684515.
- Mazur, M.; Masłowiec, D. Antimicrobial Activity of Lactones. Antibiotics 2022, 11, 1327.
- de Ruyck, J.; Dupont, C.; Lamy, E.; Le Moigne, V.; Biot, C.; Guérardel, Y.; Herrmann, J.L.; Blaise, M.; Grassin-Delyle, S.; Kremer, L.; et al. Structure-Based Design and Synthesis of Piperidinol-Containing Molecules as New Mycobacterium abscessus Inhibitors. Chem. Open 2020, 9, 351–365.
- Dias, C.; Pais, J.P.; Nunes, R.; Blázquez-Sánchez, M.-T.; Marquês, J.T.; Almeida, A.F.; Serra, P.; Xavier, N.M.; Vila-Viçosa, D.; Machuqueiro, M.; et al. Sugar-based bactericides targeting phosphatidylethanolamine-enriched membranes. Nat. Commun. 2018, 9, 4857.
- Thakur, A.; Verma, M.; Setia, P.; Bharti, R.; Sharma, R.; Sharma, A.; Negi, N.P.; Anand, V.; Bansal, R. DFT analysis and in vitro studies of isoxazole derivatives as potent antioxidant and antibacterial agents synthesized via one-pot methodology. Res. Chem. Intermed. 2023, 49, 859–883.
- Patil, S.A.; Patil, S.A.; Ble-González, E.A.; Isbel, S.R.; Hampton, S.M.; Bugarin, A. Carbazole Derivatives as Potential Antimicrobial Agents. Molecules 2022, 27, 6575.
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Antibacterial Prodrugs to Overcome Bacterial Resistance. Molecules 2020, 25, 1543.
- Bassetti, M.; Kanj, S.S.; Kiratisin, P.; Rodrigues, C.; Van Duin, D.; Villegas, M.V.; Yu, Y. Early appropriate diagnostics and treatment of MDR Gram-negative infections. JAC-Antimicrob. Resist. 2022, 4, dlac089.
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155.
- Alfei, S.; Schito, A.M. β-Lactam Antibiotics and β-Lactamase Enzymes Inhibitors, Part 2: Our Limited Resources. Pharmaceuticals 2022, 15, 476.
- Alfei, S.; Zuccari, G. Recommendations to Synthetize Old and New β-Lactamases Inhibitors: A Review to Encourage Further Production. Pharmaceuticals 2022, 15, 384.
- Karasneh, R.A.; Al-Azzam, S.I.; Ababneh, M.; Al-Azzeh, O.; Al-Batayneh, O.B.; Muflih, S.M.; Khasawneh, M.; Khassawneh, A.M.; Khader, Y.S.; Conway, B.R.; et al. Prescribers’ Knowledge, Attitudes and Behaviors on Antibiotics, Antibiotic Use and Antibiotic Resistance in Jordan. Antibiotics 2021, 10, 858.
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774.
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis. Res. 2021, 3, 7.
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344.
- Palomares, B.; Ruiz-Pino, F.; Garrido-Rodriguez, M.; Eugenia Prados, M.; Sánchez-Garrido, M.A.; Velasco, I.; Vazquez, M.J.; Nadal, X.; Ferreiro-Vera, C.; Morrugares, R.; et al. Tetrahydrocannabinolic Acid A (THCA-A) Reduces Adiposity and Prevents Metabolic Disease Caused by Diet-Induced Obesity. Biochem. Pharmacol. 2020, 171, 113693.
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150.
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346.
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628.
- Saleemi, M.A.; Yahaya, N.; Zain, N.N.M.; Raoov, M.; Yong, Y.K.; Noor, N.S.; Lim, V. Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges. Pharmaceuticals 2022, 15, 1228.
- Chen, J.; Zhang, H.; Wang, S.; Du, Y.; Wei, B.; Wu, Q.; Wang, H. Inhibitors of Bacterial Extracellular Vesicles. Front. Microbiol. 2022, 13, 835058.
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.S.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J.; et al. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. Int. J. Mol. Sci. 2023, 24, 2389.
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7.
- Gildea, L.; Ayariga, J.A.; Xu, J.; Villafane, R.; Robertson, B.K.; Samuel-Foo, M.; Ajayi, O.S. Cannabis sativa CBD Extract Exhibits Synergy with Broad-Spectrum Antibiotics against Salmonella enterica subsp. Enterica serovar typhimurium. Microorganisms 2022, 10, 2360.
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid.-Based Complement. Altern. Med. 2022, 2022, 3336516.
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473.
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259.
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, Cannabinoids, and Health. DCNS 2017, 19, 309–316.
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A New ESI-LC/MS Approach for Comprehensive Metabolic Profiling of Phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280.
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Torres-Suárez, A.I. Phyto-, Endo- and Synthetic Cannabinoids: Promising Chemotherapeutic Agents in the Treatment of Breast and Prostate Carcinomas. Expert. Opin. Investig. Drugs. 2016, 25, 1311–1323.
- Mackie, K. Cannabinoid Receptors: Where They are and What They do. J. Neuroendocr. 2008, 20, 10–14.
- Brennecke, B.; Gazzi, T.; Atz, K.; Fingerle, J.; Kuner, P.; Schindler, T.; Weck, G.; Nazaré, M.; Grether, U. Cannabinoid receptor type 2 ligands: An analysis of granted patents since 2010. Pharm. Patent Anal. 2021, 10, 111–163.
- Gertsch, J.; Raduner, S.; Altmann, K.-H. New Natural Noncannabinoid Ligands for Cannabinoid Type-2 (CB2) Receptors. J. Recept. Signal Transduct. 2006, 26, 709–730.
- Li, X.; Chang, H.; Bouma, J.; de Paus, L.V.; Mukhopadhyay, P.; Paloczi, J.; Mustafa, M.; van der Horst, C.; Kumar, S.S.; Wu, L.; et al. Structural Basis of Selective Cannabinoid CB2 Receptor Activation. Nat. Commun. 2023, 14, 1447.
- Lambert, D.M. Pharmacologic Targeting of the CB2 Cannabinoid Receptor for Application in Centrally-Mediated Chronic Pain. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2019. Available online: https://open.library.ubc.ca/collections/ubctheses/24/items/1.0376050 (accessed on 27 June 2023).
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, Related Compounds and Their Metabolic Routes. Molecules 2014, 19, 17078–17106.
- Sharma, D.S.; Paddibhatla, I.; Raghuwanshi, S.; Malleswarapu, M.; Sangeeth, A.; Kovuru, N.; Dahariya, S.; Gautam, D.K.; Pallepati, A.; Gutti, R.K. Endocannabinoid system: Role in blood cell development, neuroimmune interactions and associated disorders. J. Neuroimmunol. 2021, 353, 577501.
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (−)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638.
- Nguyen, G.N.; Jordan, E.N.; Kayser, O. Synthetic Strategies for Rare Cannabinoids Derived from Cannabis sativa. J. Nat. Prod. 2022, 85, 1555–1568.
- Schwilke, E.W.; Schwope, D.M.; Karschner, E.L.; Lowe, R.H.; Darwin, W.D.; Kelly, D.L.; Goodwin, R.S.; Gorelick, D.A.; Huestis, M.A. Δ9-Tetrahydrocannabinol (THC), 11-Hydroxy-THC, and 11-Nor-9-Carboxy-THC Plasma Pharmacokinetics during and after Continuous High-Dose Oral THC. Clin. Chem. 2009, 55, 2180–2189.
- Martin, B.R.; Jefferson, R.; Winckler, R.; Wiley, J.L.; Huffman, J.W.; Crocker, P.J.; Saha, B.; Razdan, R.K. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J. Pharmacol. Exp. Ther. 1999, 290, 1065–1079.
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.-L.; Sterner, O.; Bevan, S. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid. δ 9-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551.
- Bow, E.W.; Rimoldi, J.M. The structure–function relationships of classical cannabinoids: CB1/CB2 modulation. Perspect. Med. Chem. 2016, 8, 17–39.
- Thomas, A.; Ross, R.A.; Saha, B.; Mahadevan, A.; Razdan, R.K.; Pertwee, R.G. 6″-azidohex-2″-yne-cannabidiol: A potential neutral, competitive cannabinoid cb1 receptor antagonist. Eur. J. Pharmacol. 2004, 487, 213–221.
- O’Donnell, B.; Meissner, H.; Gupta, V. Dronabinol. In StatPearls; Updated 5 September 2022; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557531/ (accessed on 27 June 2023).
- (R)-(+)-Methanandamide. Available online: https://www.tocris.com/products/r-methanandamide_1121 (accessed on 3 May 2023).
- Gratzke, C.; Streng, T.; Stief, C.G.; Downs, T.R.; Alroy, I.; Rosenbaum, J.S.; Andersson, K.E.; Hedlund, P. Effects of cannabinor, a novel selective cannabinoid 2 receptor agonist, on bladder function in normal rats. Eur. Urol. 2010, 57, 1093–1100.
- D’Aquila, P.S. Microstructure analysis of the effects of the cannabinoid agents HU-210 and rimonabant in rats licking for sucrose. Eur. J. Pharmacol. 2020, 887, 173468.
- Ikeda, H.; Ikegami, M.; Kai, M.; Ohsawa, M.; Kamei, J. Activation of spinal cannabinoid CB2 receptors inhibits neuropathic pain in streptozotocin-induced diabetic mice. Neuroscience 2013, 250, 446–454.
- Du, J.J.; Liu, Z.Q.; Yan, Y.; Xiong, J.; Jia, X.T.; Di, Z.L.; Ren, J.J. The Cannabinoid WIN 55,212-2 Reduces Delayed Neurologic Sequelae After Carbon Monoxide Poisoning by Promoting Microglial M2 Polarization Through ST2 Signaling. J. Mol. Neurosci. MN 2020, 70, 422–432.
- Verty, A.N.; Stefanidis, A.; McAinch, A.J.; Hryciw, D.H.; Oldfield, B. Anti-Obesity Effect of the CB2 Receptor Agonist JWH-015 in Diet-Induced Obese Mice. PLoS ONE 2015, 10, e0140592.
- Howlett, A.C.; Thomas, B.F.; Huffman, J.W. The Spicy Story of Cannabimimetic Indoles. Molecules 2021, 26, 6190.
- Abadji, V.; Lin, S.; Taha, G.; Griffin, G.; Stevenson, L.A.; Pertwee, R.G.; Makriyannis, A. (R)-Methanandamide: A Chiral Novel Anandamide Possessing Higher Potency and Metabolic Stability. J. Med. Chem. 1994, 37, 1889–1893.
- WIN 55212-2. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5311501 (accessed on 3 May 2023).
- JWH-133. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6918505 (accessed on 3 May 2023).
- Hassenberg, C.; Clausen, F.; Hoffmann, G.; Studer, A.; Schürenkamp, J. Investigation of phase II metabolism of 11-hydroxy-Δ-9-tetrahydrocannabinol and metabolite verification by chemical synthesis of 11-hydroxy-Δ-9-tetrahydrocannabinol-glucuronide. Int. J. Legal Med. 2020, 134, 2105–2119.
- Engels, F.K.; de Jong, F.A.; Mathijssen, R.H.J.; Erkens, J.A.; Herings, R.M.; Verweij, J. Medicinal Cannabis in Oncology. Eu. J. Cancer 2007, 43, 2638–2644.
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol Inhibits Paclitaxel-Induced Neuropathic Pain through 5-HT1A Receptors without Diminishing Nervous System Function or Chemotherapy Efficacy. Br. J. Pharmacol. 2014, 171, 636–645.
- Keating, G.M. Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal Spray (Sativex®): A Review in Multiple Sclerosis-Related Spasticity. Drugs 2017, 77, 563–574.
- Reddy, D.S.; Golub, M.V. The Pharmacological Basis of Cannabis Therapy for Epilepsy. J. Pharmacol. Exp. Ther. 2016, 357, 45.
- Navarro, G.; Gonzalez, A.; Sánchez-Morales, A.; Casajuana-Martin, N.; Gómez-Ventura, M.; Cordomí, A.; Busqué, F.; Alibés, R.; Pardo, L.; Franco, R. Design of Negative and Positive Allosteric Modulators of the Cannabinoid CB2 Receptor Derived from the Natural Product Cannabidiol. J. Med. Chem. 2021, 64, 9354–9364.
- Luft, F.C. Rehabilitating rimonabant. J. Mol. Med. 2013, 91, 777–779.
This entry is offline, you can click here to edit this entry!
