Alzheimer’s disease (AD) represents a significant public health concern in modern society. Metabolic syndrome (MetS), which includes diabetes mellitus (DM) and obesity, represents a modifiable risk factor for AD. MetS and AD are interconnected through various mechanisms, such as mitochondrial dysfunction, oxidative stress, insulin resistance (IR), vascular impairment, inflammation, and endoplasmic reticulum (ER) stress. Therefore, it is necessary to seek a multi-targeted and safer approach to intervention. Thus, 10-hydroxy-2-decenoic acid (10-HDA), a unique hydroxy fatty acid in royal jelly, has shown promising anti-neuroinflammatory, blood–brain barrier (BBB)-preserving, and neurogenesis-promoting properties.
1. Introduction
DM is characterized by chronic hyperglycemia that results from disturbed insulin secretion or insulin dysfunction, or both [
1]. Reportedly, in 2018, a total of 440 million people worldwide were living with DM [
2], and China and India were the countries with the highest rates [
3]. It is estimated that this number will see a worldwide increase of 202 million by 2040 [
3]. According to a nationwide population-based study, individuals recently diagnosed with DM have an increased risk of developing AD after an 11-year follow-up period [
4]. Furthermore, an epidemiological study suggests that type 2 diabetes mellitus (T2DM) is a critical independent risk factor for neuropsychological symptoms, inducing anxiety, depression, and appetite disturbance in early AD [
5]. The strong association between AD and DM has led many researchers to refer to AD as “type 3 DM” [
6].
Moreover, obesity is a risk factor of T2DM. Obesity is a global public health problem, the incidence of which has tripled since 1975 [
7]. It is a health condition defined as excess body fat that endangers one’s health. In clinical practice, it is evaluated by the body mass index (BMI), calculated as weight (kg)/height
2(m
2), and is diagnosed at a BMI ≥ 30 kg/m
2 according to the WHO [
8].
AD is the primary cause of dementia, which is characterized by progressive memory loss and cognitive dysfunction. According to global data, it is estimated that 50 million people were living with dementia in 2018, and this is suggested to triple by 2050 [
9]. According to current knowledge, AD is a neuron-jeopardizing disease, which brings about a gradual loss of capacity to carry out everyday functions such as walking and gripping. Furthermore, it can lead to coma and eventually, death [
10]. All the above pose a considerable challenge to society. In addition, the risk of developing AD increases from 50% to 75% in people with DM [
11], especially T2DM [
12]. Moreover, the likelihood of a person having AD increases when their BMI is higher than average, especially if they have both obesity and poorly controlled DM [
13,
14]. Notably, T2DM and obesity originate from an unhealthy diet and lifestyle, which means that intervention through personal, clinical, and public health means is practical.
Through several interacting mechanisms, obesity and T2DM progressively cause tissue damage, particularly in the hippocampus, ultimately resulting in a decline in overall health [
15,
16,
17]. Among the mechanisms linking MetS to AD, low-grade chronic inflammation plays a critical role in the pathogenesis of AD. Importantly, low-grade inflammation disarms the BBB, making brain cells vulnerable to external stimuli [
18,
19].
Royal jelly (RJ), a white or yellowish gelatinous substance, is produced by the hypopharynx and mandibular salivary glands of honeybees [
20]. It has a long history of use in traditional Chinese medicine due to its various benefits, including its antioxidative, antimicrobial, anti-inflammatory, immunomodulatory, neurotrophic, and MetS-preventing activities [
21,
22]. Regarded as a marker of RJ quality, 10-HDA is a distinctive, unsaturated fatty acid that is present in RJ [
23], and it may be involved in the biological activities of RJ, such as antitumor and antimicrobial activities [
24,
25]. Moreover, recent research has found that 10-HDA exhibits various advantageous characteristics, such as anti-inflammatory, immunomodulatory, antimicrobial, antitumor, antioxidative, and vessel-preserving properties [
26]. These properties make 10-HDA a potentially valuable nutrient for addressing AD, particularly AD associated with MetS.
2. Anti-Neurodegeneration and Immunomodulation
The process of pyroptosis, a form of cell death associated with inflammation, has been implicated in the development of AD and MetS. The NLRP3 inflammasome is responsible for initiating pyroptosis. A study has shown that 10-HDA can enhance the function of the colonic barrier by inhibiting the NLRP3 inflammasome-mediated apoptotic pathway [
138]. Additionally, You et al. discovered that 10-HDA can alleviate neuroinflammation in microglia BV2 cells through the FOXO1-mediated autophagy pathway, indicating that it may be a promising agent for various neuroinflammation-associated diseases [
139]. Damage to the BBB is considered a crucial factor in the pathogenesis of AD. You et al. demonstrated that 10-HDA can inhibit the degradation of tight-junction proteins, reduce BBB permeability, and protect the integrity of the BBB through the AMPK/PI3K/AKT pathway [
140].
Furthermore, recent research has suggested that 10-HDA possesses immunomodulatory effects as it binds to pattern recognition receptors. One of these receptors is TLR4, which serves as a sensor for damage-associated molecular patterns [
141]. Farshid et al. found that 10-HDA acts as an antagonist to inhibit immune cell activation induced by TLR4 [
142]. These findings underscore the immunomodulatory properties of 10-HDA and its potential role in regulating immune responses.
3. Antitumor
Zafer et al. found that 10-HDA elevates the expression of caspase 3, Bax, and miR-34a. Then, it increases necrotic and apoptotic human hepatoma cells [
143]. Albalawi et al. found that 10-HDA, in combination with cyclophosphamide, showed antitumor effects against Ehrlich solid tumors [
144]. Lin et al. suggested that 10-HDA induces cell cycle arrest and apoptosis in A549 human lung cancer cells through the MAPK, STAT3, NF-κB, and TGF-β1 signaling pathways [
145]. These studies highlight the potential antitumor properties of 10-HDA and its ability to induce cell death and inhibit cancer cell growth. Properties of 10-HDA are shown in
Table 1.
Table 1. Mechanisms related to 10-HDA properties.
| Related Mechanisms |
Results |
Model |
References |
| Apoptosis |
Inhibits apoptosis in human hepatoma cells. |
Human hepatoma cell line. |
[143] |
Inflammation
Antioxidation |
Hypoglycemic effects on diabetic mice, through the PI3K/AKT/GSK3β signaling pathway. |
Diabetic C57BL/6J mice. |
[146] |
| Inflammation |
Blocks TLR4. |
HEK293T cells with high TLR4 expression. |
[142] |
Inflammation
Antioxidation |
Increases serum concentrations of immunoglobulin G at d 21, as well as IgM and interleukin-10 at d 42, while decreasing the levels of tumor necrosis factor-α. |
Broiler Chickens. |
[147] |
Inflammation
Antioxidation |
Inhibits inflammasome-mediated pyroptosis induced by LPS/ATP. |
Male C57BL/6 mice. |
[138] |
Antioxidation
Energy metabolism
Vascular function |
Maintains vascular health via scavenging •OH. |
Vascular smooth-muscle cells. |
[148] |
| Inflammation |
Attenuates the secretion of TNF-α, IL-6, and IL-1β. |
Macrophages (RAW264.7 cells) |
[149] |
| Antimicrobial |
Decreases biofilm viability and effectively eradicates mature biofilms. |
Staphylococcus aureus. |
[150] |
| Antitumor |
Decreases tumor volume, tumor markers (AFP and CEA), and TNF-α level. |
Female Swiss albino mice. |
[144] |
| Immunomodulation |
Blocks TLR4. |
Dendritic cells |
[141] |
Antimicrobial
Antioxidation |
Shows antioxidant and antimicrobial activity. |
Statens Seruminstitut Rabbit Cornea cell culture line. |
[151] |
Apoptosis
Antioxidation |
Induces apoptosis through ROS-mediated MAPK, STAT3, NF-κB, and TGF-β1 signaling pathways. |
A549 human lung cancer cells. |
[145] |
| Autophagy |
Protects against neuroinflammation through FOXO1-mediated activation of autophagy. |
Microglial BV-2 cells (LPS-induced). |
[139] |
| Immunomodulation |
Improves immunity in the thymus and spleen |
BALB/c mice. |
[152] |
| Vascular function |
Improves blood–brain barrier dysfunction by activating the AMPK/PI3K/AKT pathway. |
C57BL/6 mice (LPS-stimulated). |
[140] |
Insulin signaling
Anti-adipogenesis |
Inhibits cAMP/PKA pathway and p-Akt- and MAPK-dependent insulin signaling pathway. |
3 T3-L1 adipocyte cell line. |
[153] |
Inflammation
Antimicrobial |
Modulates interleukin-8, IL-1β, and tumor necrosis factor-alpha. |
WiDr cell. |
[154] |
| Melanogenesis inhibitor |
Inhibits the activity of tyrosinase and the expression of tyrosinase-related protein 1, TRP-2, and microphthalmia-associated transcription factor. |
B16F1 melanoma cells. |
[155] |
| Antioxidation |
Decreases tumorigenic potential of various tumor cells. |
Human colorectal adenocarcinoma cells. |
[156] |
| Insulin-like signaling |
Extends lifespan through dietary restriction signaling. |
Caenorhabditis elegans. |
[157] |
| Antioxidation |
Reduces the UVA-induced activation of the JNK and p38 MAPK pathways. |
Human dermal fibroblasts. |
[158] |
| Inflammation |
Increases procollagen type I and TGF-β1 production. |
Human dermal fibroblasts. |
[159] |
4. Metabolic Adjusting Properties
As mentioned previously, IR plays a critical role in MetS-related AD. Hu et al. found that 10-HDA increases insulin sensitivity by upregulating the PI3K/Akt pathway in the liver [
146]. In addition to adjusting glucose metabolism, Fan et al. found that 10-HDA may protect •OH-damaged vascular smooth-muscle cells by adjusting energy metabolism and protein metabolism [
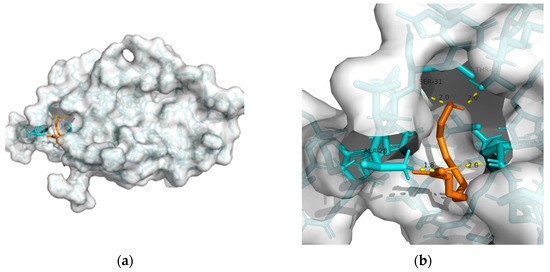
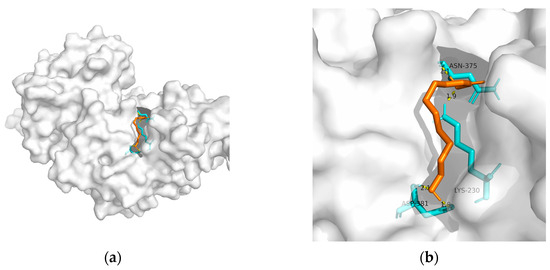
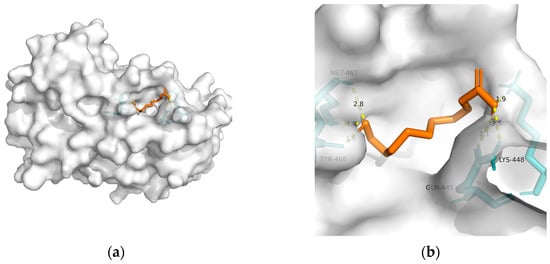
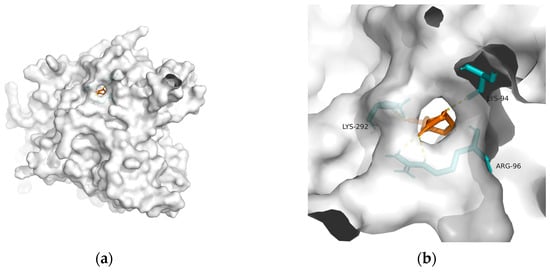
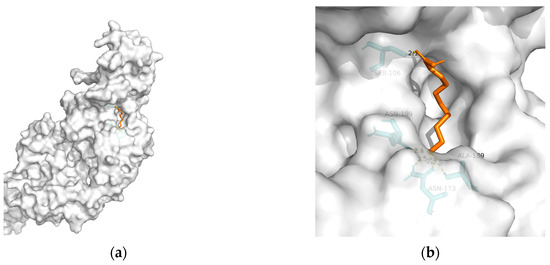
148]. Considering the above, T2DM and obesity are linked to AD through a spectrum of mechanisms, whereas no metabolic adjusting targets have been studied. Herein, several macromolecules that might be targets for MetS-related AD intervention are screened. Then, molecular docking between these macromolecules and 10-HDA is performed. The results are shown in
Table 2 and
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5.
This entry is adapted from the peer-reviewed paper 10.3390/metabo13080954