Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The cardiovascular system is a well-known target of antineoplastic treatments, including anthracyclines, chest radiotherapy and new molecules, such as tyrosine kinase inhibitors. We investigated nutritional changes in children with cancer from the diagnosis to the end of treatment and dietary habits in cancer survivors. At diagnosis, children with cancer may present variable degrees of malnutrition, potentially affecting drug tolerability and prognosis.
- childhood cancer
- cancer survivors
- antineoplastic-induced cardiotoxicity
2. Cardiovascular Damage in Cancer Patients: Acute and Chronic Cardiotoxicity
Advances in cancer therapy pursue the aim of maximizing the cure while reducing the risk of late effects [6]. Indeed, the cumulative incidence of cardiac death 15 years after cancer diagnosis is decreasing from 0.5% for those diagnosed in the 1970s to 0.1% for those diagnosed in the 1990s, as assessed by the North American Childhood Cancer Survivor Study covering over 30,000 long-term survivors of childhood cancer [14]. Nevertheless, the cumulative incidence of chronic cardiovascular disease 15 years after primary cancer diagnosis among 5-year survivors remains relevant [15] and did not significantly decrease over years for heart failure, myocardial infarction and thromboembolic disease [16]. Particularly, 30 years after anthracyclines and radiotherapy exposure, symptomatic cardiac events as congestive heart failure, cardiac ischemia, valvular disease, arrythmia and pericarditis affects one in eight cancer survivors [17].
To estimate the incidence of cardiovascular events according to different types of chemotherapy is even more difficult, varying from >20% of patients treated with anthracyclines to <5% of children receiving alkylating agents such as Cisplatin [18].
Based on time of presentation, anthracycline-related cardiotoxicity (ACT) can be categorized as acute, early and late-onset. Acute toxicity occurs in less than 1% of children within a few hours from drug infusion and its manifestations, including arrhythmias and a myocarditis-pericarditis syndrome, and are usually reversible. Early-onset toxicity usually occurs within the first year of treatment as a dilated cardiomyopathy with decreased left ventricular wall thickness and global contractility [19]. The progression of this cardiac alteration or its onset one year after the end of anthracycline therapy defines the late-onset toxicity [20]. In this case, a progressive pathologic cardiac remodeling occurs, resulting in a reduction of left ventricular dimensions and an increase of the posterior wall thickness, also described as “Grinch syndrome”. The right ventricle is less commonly affected [19].
Similarly, the cardiotoxicity related to other conventional cytotoxic chemotherapy or to newer molecularly targeted agents can be categorized in acute and early-onset if it occurs within the first year of cancer therapy or chronic cardiotoxicity if it occurs later [15].
Depending on the specific agent considered, acute toxicity described in children includes arrhythmia, transient or progressive left ventricular systolic dysfunction, myocarditis, pericarditis and vascular complications, such as hypertension and ischemia. Subclinical troponin elevation related to myocardial injury is amongst the most common effects, being observed in almost 50% of children treated with moderate dose anthracyclines for ALL [21]. In children diagnosed with acute myeloid leukemia (AML) and treated with high doses anthracyclines, left ventricular systolic dysfunction occurred in the 12% of patient with more than 70% of cases developed during the treatment [21]. The incidence of myocarditis has been estimated to be 5% of children treated with high dose cyclophosphamide [18,22]. Tyrosine kinase inhibitors may determine acute hypertension and QTc prolongation in 2–10% of children treated [15].
The prevalence of chronic cardiovascular damage is difficult to determine and may differ according to the clinical entity considered. Symptomatic cardiac dysfunction has been estimated to be around 16% among anthracyclines-exposed survivors, even though subclinical disease can occur in over 50% of children [18]. Moreover, chronic cardiomyopathy is combined with other cardiovascular alterations in 25% of childhood cancer survivors and it is associated with two or more modifiable cardiovascular risk factors in up to 10% of cases [16].
Cardiac events were found to be significantly more frequent in young survivors of cancer than in siblings and the prevalence of congestive heart failure was reported to be almost eights time higher at a mean age of 27 years (1.7% in cancer survivors versus 0.2% in siblings) [23]. Indeed, the risk of developing congestive heart failure is 4.9 times higher in children previously treated for cancer than their siblings [24]. The risk of developing valvular abnormalities and pericardial disease is respectively three times and four times higher in children treated rather than their siblings [24]. Children that develop heart failure during and/or after cancer treatment have an almost four times greater mortality risk than for children with cancer alone and the difference is even more significant when pediatric patients are compared to adults [23]. Regarding this comparison, it is difficult to assess whether children are at higher mortality risk for cardiovascular effects related to cancer treatment rather than adults. In childhood, additional comorbidities or competing causes of death are less frequent than in adults, making more dramatic the relative effect of developing heart failure. Furthermore, children are less likely to tolerate or respond to heart failure treatment and their heart could be more sensitive to the effects of cancer treatment [23].
Among the 13,060 participants in the Childhood Cancer Survivor Study, ischemic heart disease and stroke occurred in 265 and 295 childhood cancer survivors through age 50 years, respectively [25]. Radiation to the heart and exposure to anthracyclines were risk factors for ischemic heart disease, heart failure and stroke. Radiation to the heart, the brain and neck was a risk factor for stroke [25]. Furthermore, alkylating and similar DNA interstrand cross-linking such as cisplatin have been associated with an increased risk of stroke as a potential result of release of prothrombotic complexes after administration [25].
Arterial hypertension is amongst the most common cardiovascular toxicity in cancer survivors, reported with a prevalence of 37% [26], especially for newly developed target therapy against vascular endothelial growth factor (i.e., Bevacizumab), platelet-derived growth factor (i.e., Sunitinib) and fibroblast growth factor apparently in a dose-dependent manner [27].
Pericardial disease and particularly pericardial effusion and/or constriction occur in 6–30% of patients after radiation therapy. Acute pericarditis usually occurs as an acute complication of chest irradiation (within months) and it is often self-limiting whereas chronic pericarditis manifests as an effusive-constrictive disease [28].
Lastly, hematopoietic stem cell transplantation is also burdened by significant cardiotoxicity, probably as a consequence of high-doses antineoplastic treatment combined with irradiation [29]. In these patients, the cumulative incidence of coronary artery disease, cerebrovascular accident, cardiomyopathy and cardiac-related death was found to be equal to 0.2%, 0.6%, 3% and 0.5%, respectively [30].
3. Mechanisms of Antineoplastic-Induced Cardiotoxicity
The complex pathogenesis of antineoplastic-induced cardiac damage has been deeply investigated [31,32,33]. Cardiomyocytes have a limited regenerative ability that typically expose them to persistent long-term damage due to antineoplastic drugs. In the pediatric population, the effect of cardiac damage is even more complicated, varying from different drug metabolisms to intracellular diversity [28]. Unlike adults, children’s cardiomyocytes express an apoptotic pathway involving c-Myc via BAX/BAK effectors that could explain their hypersensitivity to antineoplastic drugs, specifically doxorubicin [34] (Figure 1). Indeed, a sophisticated mechanism has been described in mice, involving the mitochondrial response to genotoxic agents [34].
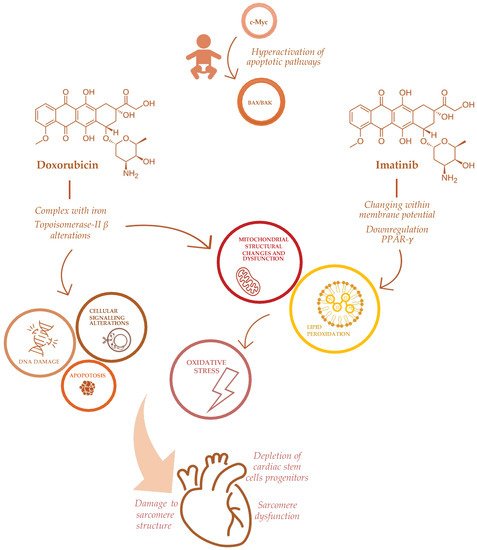
Figure 1. Mechanisms of doxorubicin and imatinib-induced cardiotoxicity in childhood cancer patients.
Among chemotherapeutic agents, anthracyclines such as doxorubicin, epirubicin, and daunorubicin remain drugs of choice for many cancer treatments, and cardiotoxicity is a well-known adverse effect of these medications [7].
ACT has been directly related to anthracyclines’ mechanism of action, involving the formation of complexes with iron, impairment of oxidation and reduction processes with subsequent increasing production of free radicals (ROS) [7]. Other effects of anthracyclines on cardiomyocytes include significant depletion of cardiac stem cells progenitors [35], a profound impairment in apoptosis and cell signaling that triggers cell death [36] and DNA synthesis [37], damaging sarcomeres’ function [38] and mitochondrial activity [7], also via Topoisomerase-II β alterations [19]. Furthermore, anthracyclines determine a direct toxicity on the sarcomeres’ structure, inhibiting calcium release from the sarcoplasmic reticulum [37], impairing formation of protein titin and the activity of mitochondrial creatine kinase [39] (Figure 1).
ACT severity can differ among patients, suggesting a different susceptibility to the cardiotoxic effects of anthracyclines. Genetic predisposition has been investigated in the pathogenesis of ACT, advocating the possible use of pharmacogenomic testing before the start of treatment [7]. In this perspective, an ACT risk prediction model, based on genetic and clinical information, has been developed [40].
Indeed, well-established risk factors for ACT are: female sex, African ancestry, trisomy 21, young age at cancer diagnosis (especially <4 years of age), chest radiation and cardiovascular risk factors [7]. Furthermore, the risk of ACT can be increased by different therapeutic strategies, such as higher anthracyclines cumulative dose (greater than 500 mg/m2), intravenous bolus injection rather than continuous infusion therapy and the use of liposomal vectors [41].
Although ACT is probably the best known antineoplastic-induced cardiotoxicity, other chemotherapeutic agents are potentially responsible for cardiovascular damage, including anti-metabolites and 5-fluorouracil, which can induce myocardial ischemia via coronary vasospasm and pericarditis [19]. Alkylating agents can lead to endomyocardial fibrosis, pericarditis, hypertension and left ventricular dysfunction, worsening ACT when combined with anthracyclines [19].
Furthermore, several emerging antineoplastic therapies, recently approved in the pediatric population, may be responsible for cardiac adverse effects. Tyrosine kinase inhibitors are novel small molecules that interfere with molecular pathways involved in cellular proliferation, differentiation and survival. Their adverse events on the cardiovascular system, hypertension, thromboembolism, pulmonary hypertension and ventricular dysfunction are described [42]. Specifically, imatinib, a first-generation tyrosine kinase inhibitor, approved for chronic myeloid leukemia, induces cardiotoxicity altering mitochondrial function through changes within the membrane potential, impairing endoplasmic reticulum response to stress, promoting apoptotic pathways and increasing reactive oxidative species production [43]. The specific mechanism of imatinib-induced cardiotoxicity is through the down-regulation of peroxisome proliferator-activated receptor-γ (PPAR-γ) levels, with a subsequent alteration in carnitine homeostasis, mitochondrial dysfunction and decreased ATP generation [43]. Through the same mechanism, imatinib seems to increase oxidative stress and the production of nitric oxidative species in vessels, thus leading to endothelial dysfunction in vivo [44] (Figure 1).
Sorafenib, an inhibitor targeting FLT3 approved for children with AML, may determine significant hypertension and left ventricular systolic dysfunction, particularly when combined with anthracyclines, due to its off-target activity on the vascular endothelial growth factor [45].
Additionally, other antineoplastic medications, such as immune checkpoint inhibitors, have been associated with rare but fatal myocarditis and arrhythmias. Finally, the novel chimeric antigen receptor T-cells therapy could induce a massive cytokine release syndrome with subsequent vasoplegic shock and ventricular dysfunction [42].
This entry is adapted from the peer-reviewed paper 10.3390/nu14163279
This entry is offline, you can click here to edit this entry!
