The presence of contaminants in the environment has increased, and studies have demonstrated that these contaminants have the ability to penetrate the blood–retinal barrier and directly affect the visual systems of organisms. Zebrafish are recognized as an ideal model for human eye diseases due to their anatomical and functional similarities to the human eye, making them an efficient and versatile organism for studying ocular toxicity caused by environmental contaminants in the field of environmental toxicology. Meanwhile, zebrafish exhibit a diverse repertoire of visually mediated behaviors, and their visual system undergoes complex changes in behavioral responses when exposed to environmental contaminants, enabling rapid assessment of the ocular toxicity induced by such pollutants.
- Zebrafish
- environmental contaminants
- ocular toxicity
- behavior
1. Introduction
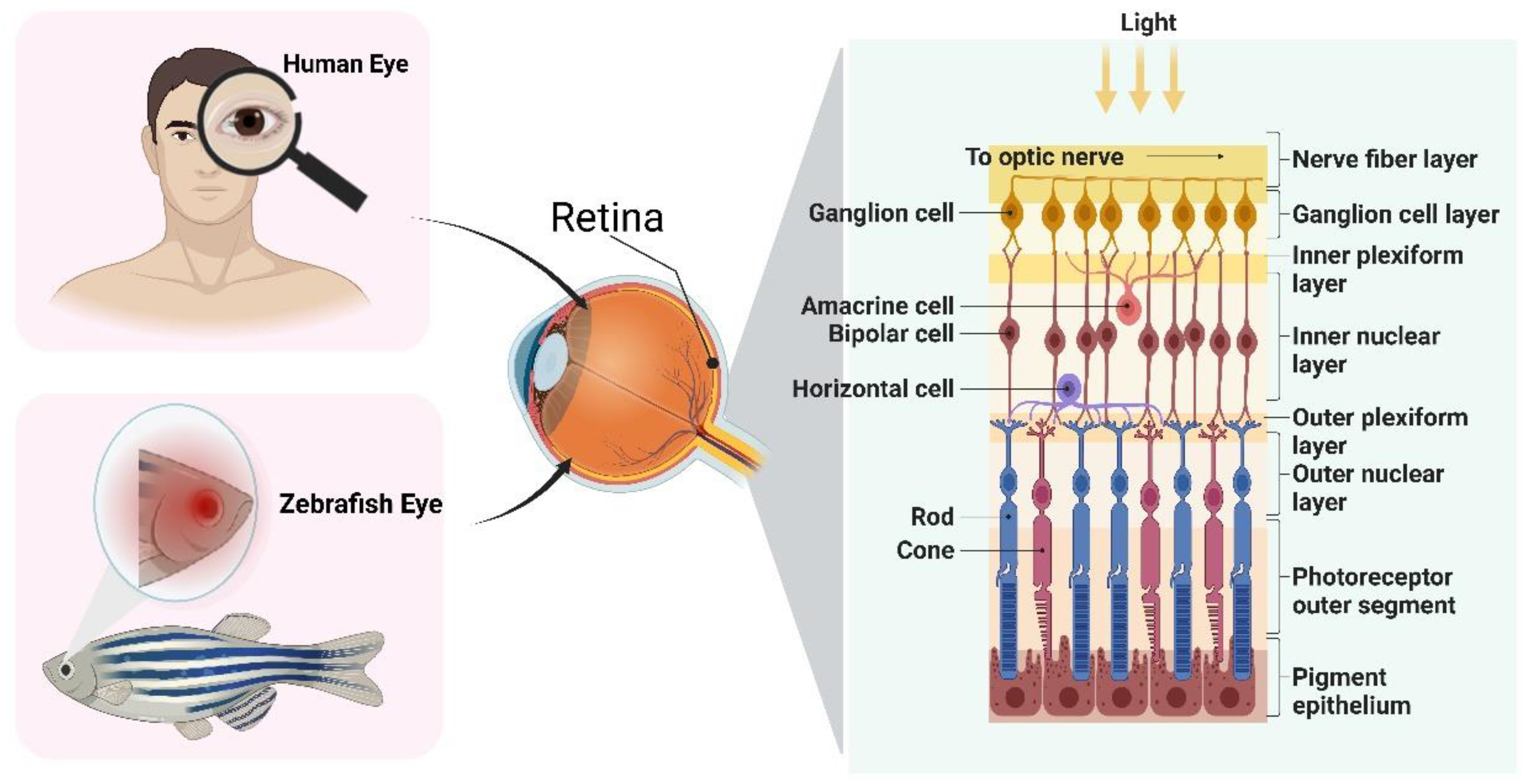
2. The Eye Structure of Zebrafish
3. Quick Approaches for Assessing Ocular Toxicity
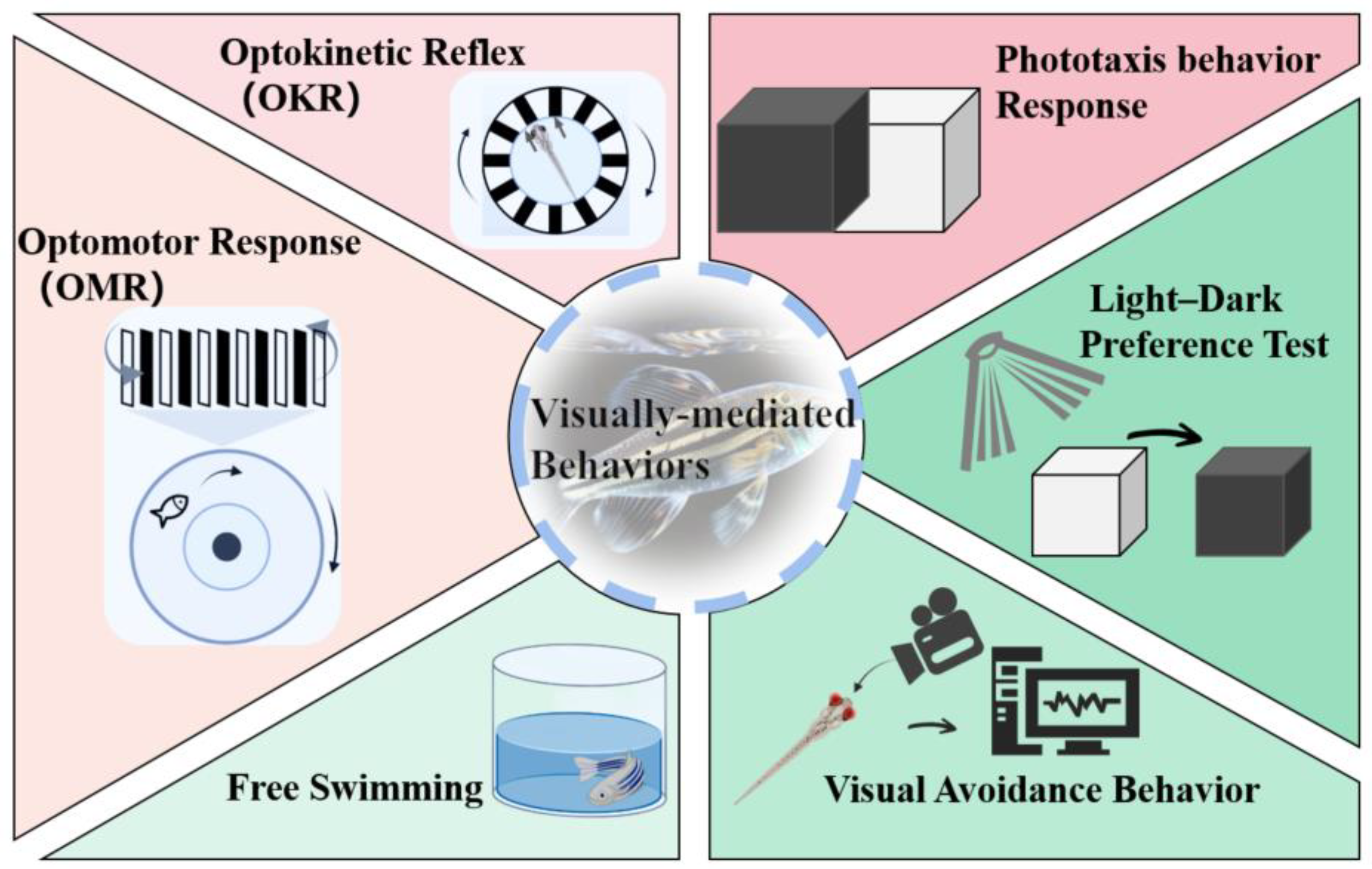
3.1. OKR
3.2. OMR
3.3. Phototaxis Behavior Response
3.4. Light-Dark Preference Test
3.5. Free Swimming
3.6. Visual Avoidance Behavior
This entry is adapted from the peer-reviewed paper 10.3390/toxics11080706
References
- Plaisancié, J.; Ceroni, F.; Holt, R.; Zazo Seco, C.; Calvas, P.; Chassaing, N.; Ragge, N.K. Genetics of Anophthalmia and Microphthalmia. Part 1: Non-Syndromic Anophthalmia/Microphthalmia. Hum. Genet. 2019, 138, 799–830.
- Zhu, S.; Gong, L.; Li, Y.; Xu, H.; Gu, Z.; Zhao, Y. Safety Assessment of Nanomaterials to Eyes: An Important but Neglected Issue. Adv. Sci. 2019, 6, 1802289.
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12.
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75.
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503.
- Bilotta, J.; Saszik, S. The Zebrafish as a Model Visual System. Int. J. Dev. Neurosci. 2001, 19, 621–629.
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23.
- Wang, L.; Ma, J.; Wu, W.; Fang, Y.; Liu, F.; Yang, Q.; Hu, X.; Gu, X.; He, Z.; Sun, D.; et al. Effect of Aerobic Exercise as a Treatment on Type 2 Diabetes Mellitus with Depression-like Behavior Zebrafish. Life Sci. 2022, 300, 120578.
- Qu, L.; Liu, F.; Fang, Y.; Wang, L.; Chen, H.; Yang, Q.; Dong, H.; Jin, L.; Wu, W.; Sun, D. Improvement in Zebrafish with Diabetes and Alzheimer’s Disease Treated with Pasteurized Akkermansia Muciniphila. Microbiol. Spectr. 2023, 11, e0084923.
- Ašmonaitė, G.; Boyer, S.; de Souza, K.B.; Wassmur, B.; Sturve, J. Behavioural Toxicity Assessment of Silver Ions and Nanoparticles on Zebrafish Using a Locomotion Profiling Approach. Aquat. Toxicol. 2016, 173, 143–153.
- Legradi, J.; el Abdellaoui, N.; van Pomeren, M.; Legler, J. Comparability of Behavioural Assays Using Zebrafish Larvae to Assess Neurotoxicity. Environ. Sci. Pollut. Res. Int. 2015, 22, 16277–16289.
- Shi, Q.; Tsui, M.M.P.; Hu, C.; Lam, J.C.W.; Zhou, B.; Chen, L. Acute Exposure to Triphenyl Phosphate (TPhP) Disturbs Ocular Development and Muscular Organization in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2019, 179, 119–126.
- Sloman, K.A.; McNeil, P.L. Using Physiology and Behaviour to Understand the Responses of Fish Early Life Stages to Toxicants. J. Fish Biol. 2012, 81, 2175–2198.
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The Zebrafish Eye—A Paradigm for Investigating Human Ocular Genetics. Eye 2017, 31, 68–86.
- Zhao, Y.; Yang, Q.; Liu, D.; Liu, T.; Xing, L. Neurotoxicity of Nanoparticles: Insight from Studies in Zebrafish. Ecotoxicol. Environ. Saf. 2022, 242, 113896.
- Shen, C.; Zuo, Z. Zebrafish (Danio Rerio) as an Excellent Vertebrate Model for the Development, Reproductive, Cardiovascular, and Neural and Ocular Development Toxicity Study of Hazardous Chemicals. Environ. Sci. Pollut. Res. Int. 2020, 27, 43599–43614.
- Cassar, S.; Dunn, C.; Ramos, M.F. Zebrafish as an Animal Model for Ocular Toxicity Testing: A Review of Ocular Anatomy and Functional Assays. Toxicol. Pathol. 2021, 49, 438–454.
- Wei, S.; Chen, F.; Xu, T.; Cao, M.; Yang, X.; Zhang, B.; Guo, X.; Yin, D. BDE-99 Disrupts the Photoreceptor Patterning of Zebrafish Larvae via Transcription Factor Six7. Environ. Sci. Technol. 2022, 56, 5673–5683.
- Lei, P.; Zhang, W.; Ma, J.; Xia, Y.; Yu, H.; Du, J.; Fang, Y.; Wang, L.; Zhang, K.; Jin, L.; et al. Advances in the Utilization of Zebrafish for Assessing and Understanding the Mechanisms of Nano-/Microparticles Toxicity in Water. Toxics 2023, 11, 380.
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early Safety Assessment of Human Oculotoxic Drugs Using the Zebrafish Visualmotor Response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8.
- Salvatore, L.; Stella, J.; Geathers, J.S.; Weber, S.R.; Grillo, M.A.; Barber, A.J.; Sundstrom, J.M.; Grillo, S.L. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells 2021, 10, 633.
- Noel, N.C.L.; Allison, W.T.; MacDonald, I.M.; Hocking, J.C. Zebrafish and Inherited Photoreceptor Disease: Models and Insights. Prog. Retin. Eye Res. 2022, 91, 101096.
- Yoshimatsu, T.; Schröder, C.; Nevala, N.E.; Berens, P.; Baden, T. Fovea-like Photoreceptor Specializations Underlie Single UV Cone Driven Prey-Capture Behavior in Zebrafish. Neuron 2020, 107, 320–337.e6.
- Wang, Y.J.; Cai, S.J.; Cui, J.L.; Chen, Y.; Tang, X.; Li, Y.H. Correlation between Photoreceptor Injury-Regeneration and Behavior in a Zebrafish Model. Neural Regen. Res. 2017, 12, 795–803.
- Hong, Y.; Luo, Y. Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals 2021, 14, 716.
- Blanco-Sánchez, B.; Clément, A.; Phillips, J.B.; Westerfield, M. Zebrafish Models of Human Eye and Inner Ear Diseases. Methods Cell Biol. 2017, 138, 415–467.
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller Cells in the Healthy and Diseased Retina. Prog. Retin. Eye Res. 2006, 25, 397–424.
- Zang, J.; Neuhauss, S.C.F. Biochemistry and Physiology of Zebrafish Photoreceptors. Pflug. Arch. 2021, 473, 1569–1585.
- Harsing, L.G.; Szénási, G.; Zelles, T.; Köles, L. Purinergic–Glycinergic Interaction in Neurodegenerative and Neuroinflammatory Disorders of the Retina. Int. J. Mol. Sci. 2021, 22, 6209.
- Shi, Q.; Wang, Z.; Chen, L.; Fu, J.; Han, J.; Hu, B.; Zhou, B. Optical Toxicity of Triphenyl Phosphate in Zebrafish Larvae. Aquat. Toxicol. 2019, 210, 139–147.
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a Better Understanding of Human Eye Disease Insights from the Zebrafish, Danio Rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330.
- Chen, L. Visual System: An Understudied Target of Aquatic Toxicology. Aquat. Toxicol. 2020, 225, 105542.
- Huang, L.; Wang, C.; Zhang, Y.; Wu, M.; Zuo, Z. Phenanthrene Causes Ocular Developmental Toxicity in Zebrafish Embryos and the Possible Mechanisms Involved. J. Hazard. Mater. 2013, 261, 172–180.
- Zeng, H.; Yang, Q.; Chen, J.; Ding, L.; Rao, F.; Lv, J.; Xie, B.; Xiang, S.; Yu, H.; Chen, X.; et al. Image-Guided Optical Coherence Tomography to Assess Structural Changes in Rodent Retinas. J. Vis. Exp. 2023, 192, e64783.
- Conedera, F.M.; Arendt, P.; Trepp, C.; Tschopp, M.; Enzmann, V. Müller Glia Cell Activation in a Laser-Induced Retinal Degeneration and Regeneration Model in Zebrafish. J. Vis. Exp. 2017, 128, 56249.
- McNeil, P.L.; Nebot, C.; Cepeda, A.; Sloman, K.A. Environmental Concentrations of Prednisolone Alter Visually Mediated Responses during Early Life Stages of Zebrafish (Danio Rerio). Environ. Pollut. 2016, 218, 981–987.
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An Emerging Model to Study Microplastic and Nanoplastic Toxicity. Sci. Total Environ. 2020, 728, 138707.
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the Move towards Ophthalmological Research. Eye 2014, 28, 367–380.
- Tao, Y.; Li, Z.; Yang, Y.; Jiao, Y.; Qu, J.; Wang, Y.; Zhang, Y. Effects of Common Environmental Endocrine-Disrupting Chemicals on Zebrafish Behavior. Water Res. 2022, 208, 117826.
- Hamilton, T.J.; Krook, J.; Szaszkiewicz, J.; Burggren, W. Shoaling, Boldness, Anxiety-like Behavior and Locomotion in Zebrafish (Danio Rerio) Are Altered by Acute BenzoPyrene Exposure. Sci. Total Environ. 2021, 774, 145702.
- Zhou, S.; Chen, Q.; Di Paolo, C.; Shao, Y.; Hollert, H.; Seiler, T.B. Behavioral Profile Alterations in Zebrafish Larvae Exposed to Environmentally Relevant Concentrations of Eight Priority Pharmaceuticals. Sci. Total Environ. 2019, 664, 89–98.
- Portugues, R.; Engert, F. The Neural Basis of Visual Behaviors in the Larval Zebrafish. Curr. Opin. Neurobiol. 2009, 19, 644–647.
- Liu, W.; Zhang, X.; Wei, P.; Tian, H.; Wang, W.; Ru, S. Long-Term Exposure to Bisphenol S Damages the Visual System and Reduces the Tracking Capability of Male Zebrafish (Danio Rerio). J. Appl. Toxicol. 2018, 38, 248–258.
- Fadool, J.M.; Dowling, J.E. Zebrafish: A Model System for the Study of Eye Genetics. Prog. Retin. Eye Res. 2008, 27, 89–110.
- Easter, S.S.; Nicola, G.N. The Development of Eye Movements in the Zebrafish (Danio Rerio). Dev. Psychobiol. 1997, 31, 267–276.
- Richards, F.M.; Alderton, W.K.; Kimber, G.M.; Liu, Z.; Strang, I.; Redfern, W.S.; Valentin, J.P.; Winter, M.J.; Hutchinson, T.H. Validation of the Use of Zebrafish Larvae in Visual Safety Assessment. J. Pharmacol. Toxicol. Methods 2008, 58, 50–58.
- Tierney, K.B. Behavioural Assessments of Neurotoxic Effects and Neurodegeneration in Zebrafish. Biochim. Biophys. Acta 2011, 1812, 381–389.
- Magnuson, J.T.; Bautista, N.M.; Lucero, J.; Lund, A.K.; Xu, E.G.; Schlenk, D.; Burggren, W.W.; Roberts, A.P. Exposure to Crude Oil Induces Retinal Apoptosis and Impairs Visual Function in Fish. Environ. Sci. Technol. 2020, 54, 2843–2850.
- Chen, X.F.; Chen, Z.F.; Lin, Z.C.; Liao, X.L.; Zou, T.; Qi, Z.; Cai, Z. Toxic Effects of Triclocarban on Larval Zebrafish: A Focus on Visual Dysfunction. Aquat. Toxicol. 2021, 241, 106013.
- Chen, L.; Huang, Y.; Huang, C.; Hu, B.; Hu, C.; Zhou, B. Acute Exposure to DE-71 Causes Alterations in Visual Behavior in Zebrafish Larvae. Environ. Toxicol. Chem. 2013, 32, 1370–1375.
- Maaswinkel, H.; Li, L. Spatio-Temporal Frequency Characteristics of the Optomotor Response in Zebrafish. Vis. Res. 2003, 43, 21–30.
- LeFauve, M.K.; Rowe, C.J.; Crowley-Perry, M.; Wiegand, J.L.; Shapiro, A.G.; Connaughton, V.P. Using a Variant of the Optomotor Response as a Visual Defect Detection Assay in Zebrafish. J. Biol. Methods 2021, 8, e144.
- Zhang, X.; Hong, Q.; Yang, L.; Zhang, M.; Guo, X.; Chi, X.; Tong, M. PCB1254 Exposure Contributes to the Abnormalities of Optomotor Responses and Influence of the Photoreceptor Cell Development in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2015, 118, 133–138.
- Zaluski, A.B.; Wiprich, M.T.; de Almeida, L.F.; de Azevedo, A.P.; Bonan, C.D.; Vianna, M.R.M. Atrazine and Diuron Effects on Survival, Embryo Development, and Behavior in Larvae and Adult Zebrafish. Front. Pharmacol. 2022, 13, 841826.
- Huang, L.; Zuo, Z.; Zhang, Y.; Wu, M.; Lin, J.J.; Wang, C. Use of Toxicogenomics to Predict the Potential Toxic Effect of Benzo(a)Pyrene on Zebrafish Embryos: Ocular Developmental Toxicity. Chemosphere 2014, 108, 55–61.
- Dehnert, G.K.; Karasov, W.H.; Wolman, M.A. 2,4-Dichlorophenoxyacetic Acid Containing Herbicide Impairs Essential Visually Guided Behaviors of Larval Fish. Aquat. Toxicol. 2019, 209, 1–12.
- Wu, L.; Zeeshan, M.; Dang, Y.; Liang, L.Y.; Gong, Y.C.; Li, Q.Q.; Tan, Y.W.; Fan, Y.Y.; Lin, L.Z.; Zhou, Y.; et al. Environmentally Relevant Concentrations of F-53B Induce Eye Development Disorders-Mediated Locomotor Behavior in Zebrafish Larvae. Chemosphere 2022, 308, 136130.
- Qian, L.; Qi, S.; Wang, Z.; Magnuson, J.T.; Volz, D.C.; Schlenk, D.; Jiang, J.; Wang, C. Environmentally Relevant Concentrations of Boscalid Exposure Affects the Neurobehavioral Response of Zebrafish by Disrupting Visual and Nervous Systems. J. Hazard. Mater. 2021, 404, 124083.
- Qiu, L.; Wei, S.; Yang, Y.; Zhang, R.; Ru, S.; Zhang, X. Mechanism of Bisphenol S Exposure on Color Sensitivity of Zebrafish Larvae. Environ. Pollut. 2023, 316, 120670.
- Comai, S.; De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; Bedini, A.; Gobbi, G. Dysfunction of Serotonergic Activity and Emotional Responses across the Light-Dark Cycle in Mice Lacking Melatonin MT2 Receptors. J. Pineal Res. 2020, 69, e12653.
- Lahouel, A.; Kebieche, M.; Lakroun, Z.; Rouabhi, R.; Fetoui, H.; Chtourou, Y.; Djamila, Z.; Soulimani, R. Neurobehavioral Deficits and Brain Oxidative Stress Induced by Chronic Low Dose Exposure of Persistent Organic Pollutants Mixture in Adult Female Rat. Environ. Sci. Pollut. Res. Int. 2016, 23, 19030–19040.
- Hwang, K.S.; Son, Y.; Kim, S.S.; Shin, D.S.; Lim, S.H.; Yang, J.Y.; Jeong, H.N.; Lee, B.H.; Bae, M.A. Size-Dependent Effects of Polystyrene Nanoparticles (PS-NPs) on Behaviors and Endogenous Neurochemicals in Zebrafish Larvae. Int. J. Mol. Sci. 2022, 23, 10682.
- Tang, Y.; Mi, P.; Li, M.; Zhang, S.; Li, J.; Feng, X. Environmental Level of the Antidepressant Venlafaxine Induces Behavioral Disorders through Cortisol in Zebrafish Larvae (Danio Rerio). Neurotoxicol Teratol. 2021, 83, 106942.
- Wang, X.H.; Souders, C.L.; Xavier, P.; Li, X.Y.; Yan, B.; Martyniuk, C.J. The Pyrethroid Esfenvalerate Induces Hypoactivity and Decreases Dopamine Transporter Expression in Embryonic/Larval Zebrafish (Danio Rerio). Chemosphere 2020, 243, 125416.
- Maharaj, S.; El Ahmadie, N.; Rheingold, S.; El Chehouri, J.; Yang, L.; Souders, C.L.; Martyniuk, C.J. Sub-Lethal Toxicity Assessment of the Phenylurea Herbicide Linuron in Developing Zebrafish (Danio Rerio) Embryo/Larvae. Neurotoxicol. Teratol. 2020, 81, 106917.
- Zhao, J.; Xu, T.; Yin, D.Q. Locomotor Activity Changes on Zebrafish Larvae with Different 2,2′,4,4′-Tetrabromodiphenyl Ether (PBDE-47) Embryonic Exposure Modes. Chemosphere 2014, 94, 53–61.
- Wang, Y.; Chen, J.; Du, C.; Li, C.; Huang, C.; Dong, Q. Characterization of Retinoic Acid-Induced Neurobehavioral Effects in Developing Zebrafish. Environ. Toxicol. Chem. 2014, 33, 431–437.
- Wei, S.; Qiu, L.; Ru, S.; Yang, Y.; Wang, J.; Zhang, X. Bisphenol S Disrupts Opsins Gene Expression and Impairs the Light-Sensing Function via Antagonizing TH-TRβ Signaling Pathway in Zebrafish Larvae. Food Chem. Toxicol. 2023, 172, 113588.
- Sehonova, P.; Hodkovicova, N.; Urbanova, M.; Örn, S.; Blahova, J.; Svobodova, Z.; Faldyna, M.; Chloupek, P.; Briedikova, K.; Carlsson, G. Effects of Antidepressants with Different Modes of Action on Early Life Stages of Fish and Amphibians. Environ. Pollut. 2019, 254, 112999.
- Li, D.; Sun, W.; Chen, H.; Lei, H.; Li, X.; Liu, H.; Huang, G.-Y.; Shi, W.J.; Ying, G.G.; Luo, Y.; et al. Cyclophosphamide Affects Eye Development and Locomotion in Zebrafish (Danio Rerio). Sci. Total Environ. 2022, 805, 150460.
- Ivantsova, E.; Konig, I.; Souders, C.L.; McNabney, D.; Simmons, D.D.B.; Martyniuk, C.J. Lipidomic, Metabolomic, and Behavior Responses of Zebrafish (Danio Rerio) Exposed to Environmental Levels of the Beta Blocker Atenolol. Sci. Total Environ. 2023, 866, 161272.
- Saint-Amant, L.; Drapeau, P. Time Course of the Development of Motor Behaviors in the Zebrafish Embryo. J. Neurobiol. 1998, 37, 622–632.
- Thiagarajan, V.; Alex, S.A.; Seenivasan, R.; Chandrasekaran, N.; Mukherjee, A. Interactive Effects of Micro/Nanoplastics and Nanomaterials/Pharmaceuticals: Their Ecotoxicological Consequences in the Aquatic Systems. Aquat. Toxicol. 2021, 232, 105747.
- Gaylarde, C.C.; Baptista Neto, J.A.; da Fonseca, E.M. Nanoplastics in Aquatic Systems—Are They More Hazardous than Microplastics? Environ. Pollut. 2021, 272, 115950.
- Liu, Y.; Wang, Y.; Ling, X.; Yan, Z.; Wu, D.; Liu, J.; Lu, G. Effects of Nanoplastics and Butyl Methoxydibenzoylmethane on Early Zebrafish Embryos Identified by Single-Cell RNA Sequencing. Environ. Sci. Technol. 2021, 55, 1885–1896.
- Liu, Y.; Wang, Y.; Li, N.; Jiang, S. Avobenzone and Nanoplastics Affect the Development of Zebrafish Nervous System and Retinal System and Inhibit Their Locomotor Behavior. Sci. Total Environ. 2022, 806, 150681.
- Ranasinghe, P.; Thorn, R.J.; Seto, R.; Creton, R.; Bridges, W.C.; Chapman, S.C.; Lee, C.M. Embryonic Exposure to 2,2′,3,5′,6-Pentachlorobiphenyl (PCB-95) Causes Developmental Malformations in Zebrafish. Environ. Toxicol. Chem. 2020, 39, 162–170.
- Santos, D.; Luzio, A.; Matos, C.; Bellas, J.; Monteiro, S.M.; Félix, L. Microplastics Alone or Co-Exposed with Copper Induce Neurotoxicity and Behavioral Alterations on Zebrafish Larvae after a Subchronic Exposure. Aquat. Toxicol. 2021, 235, 105814.
