Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Integrative & Complementary Medicine
Chronic inflammatory respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis, present challenges in terms of effective treatment and management. These diseases are characterized by persistent inflammation in the airways, leading to structural changes and compromised lung function. To achieve optimal therapeutic outcomes while minimizing systemic side effects, targeted therapies and precise drug delivery systems are crucial to the management of these diseases.
- drug delivery
- chronic inflammatory respiratory diseases
- nanoparticle-based drug delivery systems
- inhaled corticosteroids
1. Introduction
Chronic inflammatory respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), affect millions of people worldwide and are a leading cause for the increase in lung disease morbidity and mortality [1]. Asthma, as a heterogeneous clinical syndrome, affects over 300 million people worldwide [2]. COPD, a disease mainly associated with long-term smoking, became the third leading cause of death globally in 2020 [3]. Although there are several existing treatments, limited efficacy, side effects, and individual variability still cannot be ignored [4,5,6]. In recent years, there has been a growing interest in the development of targeted drug delivery systems for the treatment of these diseases [7,8,9]. Nanoparticle-based drug delivery systems, inhaled corticosteroids (ICSs), novel biologicals, gene therapy, and personalized medicine have emerged as promising approaches to deliver drugs more effectively and with fewer side effects.
Currently, the development of new nanoparticle-based drug delivery systems that can target specific cells such as lung epithelial cells and macrophages, while minimizing systemic side effects, have received significant attention [10]. These systems utilize nanoparticles, which are tiny particles ranging from 1 to 100 nanometers in size, to encapsulate and deliver drugs directly to the affected areas of the lungs [11]. By modifying the surface properties of nanoparticles, researchers can enhance their ability to selectively bind to specific cell types in the lungs, thereby improving drug delivery efficiency and reducing off-target effects [12]. Furthermore, nanoparticle-based drug delivery systems can protect the drugs from degradation and enhance their stability, ensuring sustained release and prolonged therapeutic effects [13].
In addition to nanoparticle-based systems, inhaled corticosteroids (ICSs) have long been used as a standard treatment for chronic inflammatory respiratory diseases [14,15]. ICSs work by reducing inflammation in the airways, thus alleviating symptoms and preventing exacerbation. Researchers are also exploring novel biological targets and innovative methods for delivering biologicals to the lungs. Gene therapy approaches, including viral-vector-based delivery systems and CRISPR–Cas9 technology, represent another exciting frontier in the treatment of chronic inflammatory respiratory diseases [16,17]. Moreover, personalized medicine approaches take into account an individual’s unique characteristics, such as genetics, biomarkers, and lifestyle factors, to tailor treatments to their specific needs [8,18]. By utilizing advanced diagnostic tools like genomic sequencing and biomarker analysis, healthcare providers can identify patient subgroups who are more likely to respond to a particular therapy, thus optimizing treatment outcomes [19,20]. However, several challenges remain, including optimizing delivery efficiency, ensuring safety, and addressing ethical considerations.
2. Nanoparticle-Based Drug Delivery Systems
The application of nanotechnology continues to provide effective strategies in treating various chronic diseases and improving treatment outcomes. Using nanocarrier systems such as liposomes, micelles, and nanoparticles for pulmonary drug delivery has been proven to be a promising noninvasive treatment strategy for achieving drug deposition and controlled release in the lungs [10] (Figure 1). These systems involve the use of engineered particles with dimensions on the nanometer scale to deliver drugs directly to target cells in the lungs [21]. Nanoparticles have several advantages over conventional drug delivery methods, including improved bioavailability, enhanced targeting, and reduced toxicity [22,23].
Figure 1. Nanocarrier systems can achieve drug deposition and controlled release in the lungs.
Liposomes are spherical vesicles composed of lipid bilayers that can encapsulate both hydrophilic and hydrophobic drugs [24]. The size, surface charge, and lipid composition of lipid nanoparticles (LNPs) can be tailored to enhance drug stability, prolong circulation time, and improve biocompatibility [25]. Furthermore, conjugating small-molecule ligands, peptides [26], or monoclonal antibodies [27] to the surface of an LNP can endow it with targeting ability. For example, folate receptors are often found to be overexpressed on macrophages, which makes folate-coupled LNP a great option for delivering anti-inflammatory drugs [28]. There are many factors that can affect the release of cargo carried by LNPs, including temperature, changes in pH values, enzymes, light, etc. Among them, the mechanism of pH change is the most studied, and can cause LNPs to undergo phase transition and achieve higher membrane permeability [29].
In addition to LNPs, there are also some other nanoparticles that have their own characteristics (Table 1). Micelles are another kind of nanoparticle consisting of amphiphilic molecules that form a core-shell structure [30]. Their great solubility allows them to easily penetrate the increased alveolar fluid barrier present in chronic inflammatory lung diseases. A new kind of stabilized phospholipid nanomicelles (SSMs) can reach deep lung tissue and successfully extend the half-life of budesonide in the lung to 18–20 h [31]. Magnetic nanoparticles (MNPs) developed using the magnetofection technique have wide-ranging applications in the fields of biological research and medicine, including drug and gene therapy, magnetic targeting (such as in cancer therapies), and diagnostic imaging as contrast enhancers [32,33]. A representative example is the superparamagnetic iron oxide nanoparticle (SPION), a type of nanoparticle with special magnetism that can be guided through an external magnetic field to locations within the body [34]. They can accurately transport the drugs coated on their surface, mainly some inflammation-related molecular antibodies like IL4Rα and ST2, to the site of the inflammatory lesion [35,36]. A kind of selective organ targeting (SORT) nanoparticle was designed to release its cargo in a controlled manner; it can target the site of inflammation in the lungs and elsewhere while minimizing exposure of healthy tissue in other parts of the body [37]. This targeted drug delivery approach has the potential to reduce drug toxicity and improve patient outcomes [38]. Recently, a growing number of hybrid nanoparticles (HNPs) have emerged that can simultaneously possess the characteristics of different nanoparticles [39]. This has sparked a trend of exploring different combinations of nanoparticles.
Table 1. Therapeutic applications of nanoparticles in chronic inflammatory respiratory diseases.
| Diseases | Type of Nanoparticles | Drugs | Target Ligands | Targets | References |
|---|---|---|---|---|---|
| Asthma | SPION | None | IL4Rα monoclonal antibody | ASMs | [35] |
| SPION | None | Anti-ST2 blocking antibodies | Inflammatory lung tissue | [36] | |
| PLGA-based nanoparticles | Smart silencer of Dnmt3aos | Exosome membrane of M2 macrophages | M2 macrophages | [40] | |
| LNP | Polyinosinic-polycytidylic acid | None | Lung epithelial cells |
[41] | |
| COPD | HNP | siRNA against SCNN1A and SCNN1B | None | Lung epithelial cells |
[42] |
| LNP | siRNA against TNF-α | None | None | [43] | |
| IPF | LNP | siRNA against IL-11 | None | MLFs | [44] |
| CF | LNP | Plasmid DNA | ICAM-1 targeting peptide |
Lung epithelial cells |
[45] |
Abbreviations: SPION: Superparamagnetic iron oxide nanoparticle; IL4Rα: Interleukin-4 receptor alpha; ASM: Airway smooth muscle cell; ST2: Grow stimulation expressed gene 2; PLGA: Polylactic-co-glycolic acid; LNP: Lipid nanoparticle; HNP: Hybrid nanoparticle; SCNN1A: Sodium channel non-alpha subunit 1A; SCNN1B: Sodium channel non-alpha subunit 1B; TNF-α: Tumor necrosis factor alpha; IL-11: Interleukin-11; MLFs: Mouse lymphatic fibroblasts; ICAM-1: Intercellular adhesion molecule-1.
Despite the promise of nanoparticle-based drug delivery, there are still several research challenges that need to be addressed. For example, there is a need to develop nanoparticles with optimal physicochemical properties, such as particle size, surface charge, and stability, to ensure effective drug delivery [46]. Recent research has reported that the structure of mesoporous silica nanoparticles (MSNs) can be well controlled with several parameters such as pH, surfactant, silica precursor, and temperature. For instance, Pan et al. prepared a series of size-controlled MSNs with a range of 25–105 nm by simply changing the amount of the basic catalyst triethanolamine (TEA) added [47]. So, it is believed that MSNs have significant potential to serve as nanocarriers for pulmonary drug delivery [48]. Additionally, researchers need to carefully evaluate the safety and toxicity of nanoparticle-based drug delivery systems. While some studies have shown promising results, others have raised concerns about the potential for long-term toxicity and negative environmental impacts of nanoparticle-based drug delivery [49,50]. Currently, it is widely believed that the cytotoxicity of nanoparticles is mainly related to their large surface area and small size [51]. Yuan et al. concluded through their study on the effects of 20, 30, and 40 nm zinc oxide nanoparticles on human embryonic lung fibroblasts that cytotoxicity is concentration-dependent, therefore calling for the minimum therapeutic concentration [52]. Other researchers found that the surface charge and solubility are also associated with the cytotoxicity of nanoparticles [53,54].
Moving forward, researchers are exploring several future directions for nanoparticle-based drug delivery systems. For example, considering that there is a large amount of mucus oozing out of the lungs during chronic inflammatory diseases, researchers are developing new mucus-penetrating nanoparticles (MPPs). Uptake mechanism studies revealed that caveolae-mediated endocytosis and macropinocytosis contributed to the absorption of MPPs [55]. In vivo research results showed a more than five-fold increase in drug bioavailability [56]. Others are investigating new methods for optimizing nanoparticle design and surface modification to improve targeting and drug release [40,57]. Additionally, some researchers are investigating the potential of combining nanoparticles with other treatment modalities such as gene therapy or immunotherapy [46,58]. Finally, there is growing interest in developing personalized nanoparticle-based drug delivery approaches that can be tailored to individual patients based on their unique disease characteristics and genetic profiles [59].
Through targeted drug delivery, nanoparticles have the potential to improve therapeutic efficacy and reduce systemic side effects. Overall, nanoparticle-based drug delivery systems hold great promise for the treatment of chronic inflammatory respiratory diseases.
3. Inhaled Corticosteroids (ICSs)
Inhaled corticosteroids (ICSs) are widely used as a treatment option for chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). These medications work by reducing the production of inflammatory mediators in the airways, which helps prevent or reduce inflammation, bronchoconstriction, and mucus production. According to the Global Initiative for Asthma (GINA) report [1], ICSs have been shown to improve lung function, reduce exacerbation, and improve quality of life in patients with chronic respiratory diseases.
However, there are some current challenges with ICS delivery that limit their efficacy. One major challenge is achieving the optimal distribution of the medication throughout the lungs. ICS particles can become trapped in the mouth or throat, reducing their effectiveness in the lower airways [60]. Patients may also have difficulty using their inhaler correctly, leading to reduced medication delivery and efficacy [61]. Moreover, selecting the appropriate ICS dose for each patient can be challenging, as individual needs can vary significantly [62].
To optimize ICS delivery and improve its efficacy, several methods have been developed. One approach involves the use of spacer devices, which help to slow down the speed of medication delivery and improve medication deposition in the lungs [63]. Another approach is the development of more efficient ICS formulations, such as fine-particle ICSs, which have shown improved efficacy compared with conventional ICS formulations [64]. Fine-particle ICSs have greater deposition in the small airways compared with conventional ICSs [65]. According to a meta-analysis, fine-particle ICSs have significantly higher odds of achieving asthma control [66]. The combination of ICSs and other drugs is also worth further optimization (Figure 2). Additionally, research advancements have explored smart inhalers that can monitor medication adherence and provide feedback to patients [67]. Nowadays, four kinds of inhalers (nebulizers, dry powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs), and soft mist inhalers (SMIs)) are widely used (Table 2). Recently, artificial intelligence (AI)-based intelligent inhalers have attracted much attention, as they can enable real-time regulation of inhalation plans. For example, intelligent dry powder inhalers (DPIs) constructed based on artificial neural networks (ANNs) have effectively improved the bioavailability of drugs [68], but additional data are still needed to train more advanced models to output better drug delivery plans [69].
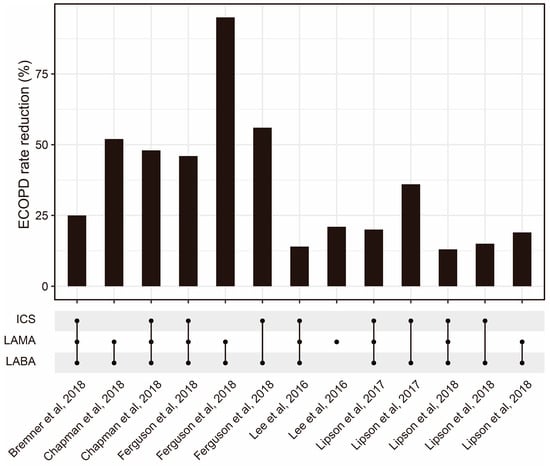
Figure 2. The ECOPD rate reduction from ICSs combined with other drug regimens reported by some published studies [70,71,72,73,74,75]. Abbreviations: ECOPD: Exacerbation of chronic obstructive pulmonary disease; ICS: Inhaled corticosteroid; LAMA: Long-acting muscarinic antagonist; LABA: Long-acting beta2-adrenergic agonist.
Table 2. Different kinds of ICS inhalers.
| Type of Inhaler | Subtype | Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Nebulizers | Jet (or pneumatic) | Use compressed air or oxygen to convert liquid medication into a fine mist for inhalation. | Versatile and suitable for all ages. | Longer administration times, produce noise and vibration, require power sources, and need regular maintenance. | [76] |
| Ultrasonic | Use high-frequency vibrations to convert liquid medication into a fine mist for inhalation. | Portable and compact, have faster administration times, operate quietly. | Not suitable for medications that are heat-sensitive or contain suspensions. | [77] | |
| Mesh | Use a vibrating mesh or perforated plate to generate a fine aerosol mist from liquid medication. | Portable, lightweight, and operate silently with faster administration times. | Have limitations in delivering higher viscosity medications or large medication volumes. | [78] | |
| Dry powder inhalers (DPIs) | Single- and multi-unit doses | Deliver medication directly to the lungs in a powdered form. | Breath-activated, portable, and do not require coordination between inhalation and device activation. | Require adequate inspiratory flow for optimal drug delivery, and can be used only with specific types of dry powder medications. | [79] |
| Pressurized metered-dose inhalers (pMDIs) | Single and combined drugs | Deliver medication in a pressurized aerosol form using propellants. | Deliver a consistent dose, require minimal preparation time. | The presence of propellants and the inability to assess remaining medication levels easily. | [80] |
| Soft mist inhalers (SMIs) | None | Deliver medication as a slow-moving aerosol mist. | Provide consistent and precise dosing, generate a slow-moving mist suitable for patients with diverse inspiratory abilities, and are equipped with dose counters to monitor medication levels. | Potential clogging if not used properly, higher cost compared with other inhalers, and limited availability of medications in soft mist formulation. | [81] |
While there have been notable advancements, it is important to acknowledge that there are still existing limitations concerning the use of ICSs that necessitate careful consideration and remediation. For example, some studies have suggested that long-term use of ICSs may increase the risk of pneumonia and cataracts [82,83]. Moreover, further research is needed to determine the optimal ICS dose and duration of treatment for individual patients [84].
Future directions for research in ICS delivery are focused on several areas. Personalized ICS dosing strategies based on individual patient characteristics and disease severity are being explored [85]. Investigations are currently underway to explore new ICS formulations that utilize innovative drug delivery technologies, including nanotechnology and microencapsulation [86].
Thus, ICSs remain an effective treatment option for chronic respiratory diseases, but proper delivery optimization is crucial to their efficacy and safety.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15082151
This entry is offline, you can click here to edit this entry!
