Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a revolutionary genome editing technology that allows the precise modification of DNA sequences. Liposomes, which are small vesicles composed of lipid bilayers, have emerged as promising carriers for delivering various therapeutic agents, including CRISPR components.
- liposome
- CRISPR/Cas9
- single-guide RNA
- gRNA
1. Introduction
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a revolutionary genome editing technology that allows the precise modification of DNA sequences. It has been widely used by scientists and offers great potential for gene therapy for various diseases. CRISPR/Cas9 is one of the most promising major advances in the field of gene therapy [1][2][3]. The CRISPR–Cas9 system consists of a guide RNA (gRNA) and the Cas9 nuclease [4][5][6][7][8]. The Cas9 protein is the enzyme that acts as the molecular scissors in the CRISPR–Cas9 system, responsible for cutting the DNA strands. The guide RNA (gRNA) is a small RNA molecule. It binds to the Cas9 protein and guides it to the desired location in the genome. Once the Cas9 protein reaches the target gene, it creates a precise cut or break in both strands of the DNA helix. This break triggers the cell’s DNA repair machinery. The DNA repair mechanisms that come into play after the double-strand break can result in different outcomes [9][10][11].
Efficient and targeted delivery of CRISPR components is crucial for successful genome editing. To achieve the desired genetic modifications, Cas9 and guide RNA molecules must be delivered to the specific cells or tissues of interest. However, delivering large biomolecules like CRISPR components into cells is challenging due to their sizes and the natural barriers of the cell membrane. Methods for delivering CRISPR components into cells can be broadly categorized into three types: physical transfection, viral transfection, and non-viral chemical transfection. Physical transfection involves creating temporary pores in the cell membrane, allowing gRNA/Cas9 to enter the cell. Three common physical methods used for introducing CRISPR components into cells are electroporation, nucleofection, and microinjection. Viral transfection utilizes viral vectors to transfer DNA or RNA into cells through a process called transduction. Several types of viruses can be used for transduction, including lentiviruses, adenoviruses, adeno-associated viruses, and herpes viruses. These viruses are engineered to carry the desired CRISPR components and efficiently deliver them into the target cells. Chemical transfection utilizes various chemical compounds to transport molecules into cells, including calcium phosphates, cationic polymers, and cationic amino acids. One of the commonly employed chemical transfection methods for introducing CRISPR components into cells is lipofection using liposomes [12][13][14].
Liposomes, which are small vesicles composed of lipid bilayers, have emerged as promising carriers for delivering various therapeutic agents, including CRISPR components. Their unique structure and properties make liposomes ideal for encapsulating and protecting biomolecules while also facilitating their delivery into target cells.
2. Liposome-Based Delivery System
Liposomes, stable spherical vesicles made of cholesterol and nontoxic phospholipids, have attracted attention and found diverse applications due to their amphiphilic nature, biocompatibility, biodegradability, and facile surface modification. They represent versatile carriers for drug and gene delivery, offering numerous advantages including encapsulation, targeted delivery, protection from degradation and metabolism, controlled release, and biocompatibility. Their ability to overcome biological barriers and enhance drug efficacy makes them an attractive option for the development of novel pharmaceutical formulations [15][16][17][18][19][20]. In the field of gene therapy, liposomes have played a significant role because they can encapsulate and deliver genetic material, such as DNA or RNA, to target cells [21][22][23].
Over the past 50 years, liposome-based gene delivery has achieved significant milestones (Figure 1). Originally discovered in the 1960s [24], the concept of using liposomes as carriers for gene delivery originated in the 1980s [25][26][27]. Despite early challenges of low transfection efficiency, liposome formulations have been developed to deliver siRNA, plasmid DNA, and mRNA for gene silencing, replacement, and protein expression [25][26][27][28][29][30][31][32][33][34]. Surface modifications of liposomes with ligands, such as antibodies or peptides, have been a focus of research, enabling selective binding to specific receptors on target cells and improving delivery efficiency [12][13][35][36][37][38]. Additionally, stimuli-responsive liposomes were engineered to release their cargo in response to environmental cues, such as pH changes or ultrasound activity [37][39][40][41]. Clinical trials have extensively evaluated liposome-based gene delivery systems, demonstrating positive results for the treatment of diseases [15][16][42]. In recent years, lipid-based nanotechnology has demonstrated significant advancements, offering innovative approaches for gene delivery and therapeutics [43][44][45][46][47]. Surface modifications, stimuli-responsive liposomes, and clinical trials have further advanced this field. Escaping endosomes remains a challenge, but optimized gene loading, stability, and controlled release may hold promise for increased genome editing efficiency [35][36][40][41][42][43][44][45][46][47].
Figure 1. Schematic illustration of the development of liposome-based gene delivery.
The preparation process involves formulating liposomes with cationic lipids and genetic material, forming complexes for administration to target cells. PEGylation (Polyethylene Glycosylation) is a technique employed to modify the desired material by attaching polyethylene glycol (PEG) chains to them. PEG can improve the pharmacokinetic properties of therapeutic agents through increased half-lives, reduced immunogenicity, enhanced solubility and stability, and improved tissue penetration [48][49]. Once at the target site, liposomes interact with cell membranes and release genetic material for therapeutic genome editing. Liposomes present advantages for CRISPR delivery, ensuring stability, enhanced cellular uptake, and targeting through surface modifications.
3. Liposome-Based CRISPR Delivery
Liposomes have been extensively engineered to optimize the delivery of CRISPR/Cas and gRNA [12][13][35][36][37][40][50][51][52]. The combination of the CRISPR-based system with liposome delivery technology enables precise and efficient genetic modifications in cells and tissues. In the context of the CRISPR combination approach, liposomes are modified to carry CRISPR components, such as Cas9 and gRNA.
CRISPR has been extensively utilized for making accurate and precise modifications to the genetic code [4][5][6][7][8]. It utilizes gRNA to target a specific DNA sequence, which is then recognized and cleaved by an enzyme called Cas9. Subsequently, the cell’s own DNA repair mechanisms can be harnessed to introduce desired genetic modifications. CRISPR has shown remarkable potential in gene therapy, exhibiting promising results in treating conditions like sickle cell anemia and cystic fibrosis [1][2][3]. However, the effectiveness of the CRISPR/Cas system has been limited by the lack of efficient delivery methods. An essential prerequisite for successful gene editing is the development of a delivery strategy that can effectively deliver the CRISPR cargo to the target for effect.
Liposomes, made of lipids like cell membranes, can encapsulate diverse agents (e.g., hydrophobic/hydrophilic molecules, proteins, peptides, and nucleic acids) [42][53][54][55][56]. By modifying their surface with ligands or antibodies that recognize specific receptors or markers on the target cells, liposomes can be targeted to specific cells or tissues thereby enhancing the specificity and efficacy of the therapy while minimizing off-target effects. Furthermore, liposomes can be designed to respond to specific environmental cues, such as pH, temperature, or other stimuli, to trigger payload release at the desired location [42][55][56]. Overall, liposomes have shown great promise as CRISPR delivery.
3.1. Enhanced Targeting and Cellular Uptake In Vitro and In Vivo
Liposome-based vectors provide a versatile and efficient means of delivering CRISPR components into cells for genome editing in vitro [13][36][37][57]. The liposomes can fuse with the cell membrane or be internalized via endocytosis, facilitating the release of the encapsulated CRISPR components into the cell’s cytoplasm. Before entering the nucleus to induce gene editing, the Cas9 enzyme and gRNA form a complex. The gRNA guides the Cas9 enzyme to the specific target site in the genome by binding it to the complementary DNA sequence. The use of liposomes as delivery vehicles presents their biocompatibility, protection from degradation, and enhanced cellular uptake [58][59].
Liposome-based vectors have exhibited the ability for in vivo CRISPR-based genome editing, delivering CRISPR components into living organisms for precise modifications in specific cells or tissues [40][51]. The liposomes are typically safe for use in living organisms and can be modified to optimize their stability and targeting capabilities. Also, they can be engineered to incorporate ligands or surface modifications that allow specific recognition and uptake by target cells, enhancing the delivery efficiency. The effectiveness of liposome-based vectors for in vivo CRISPR-based genome editing depends on several factors, including the choice of lipids, the efficiency of encapsulation, the stability of the liposomes in the physiological environment, and the specificity and efficacy of targeting the desired cells or tissues.
CRISPR/Cas9 components can be effectively delivered to cells and/or tissues using liposome-based vectors and delivery methods (Figure 2).
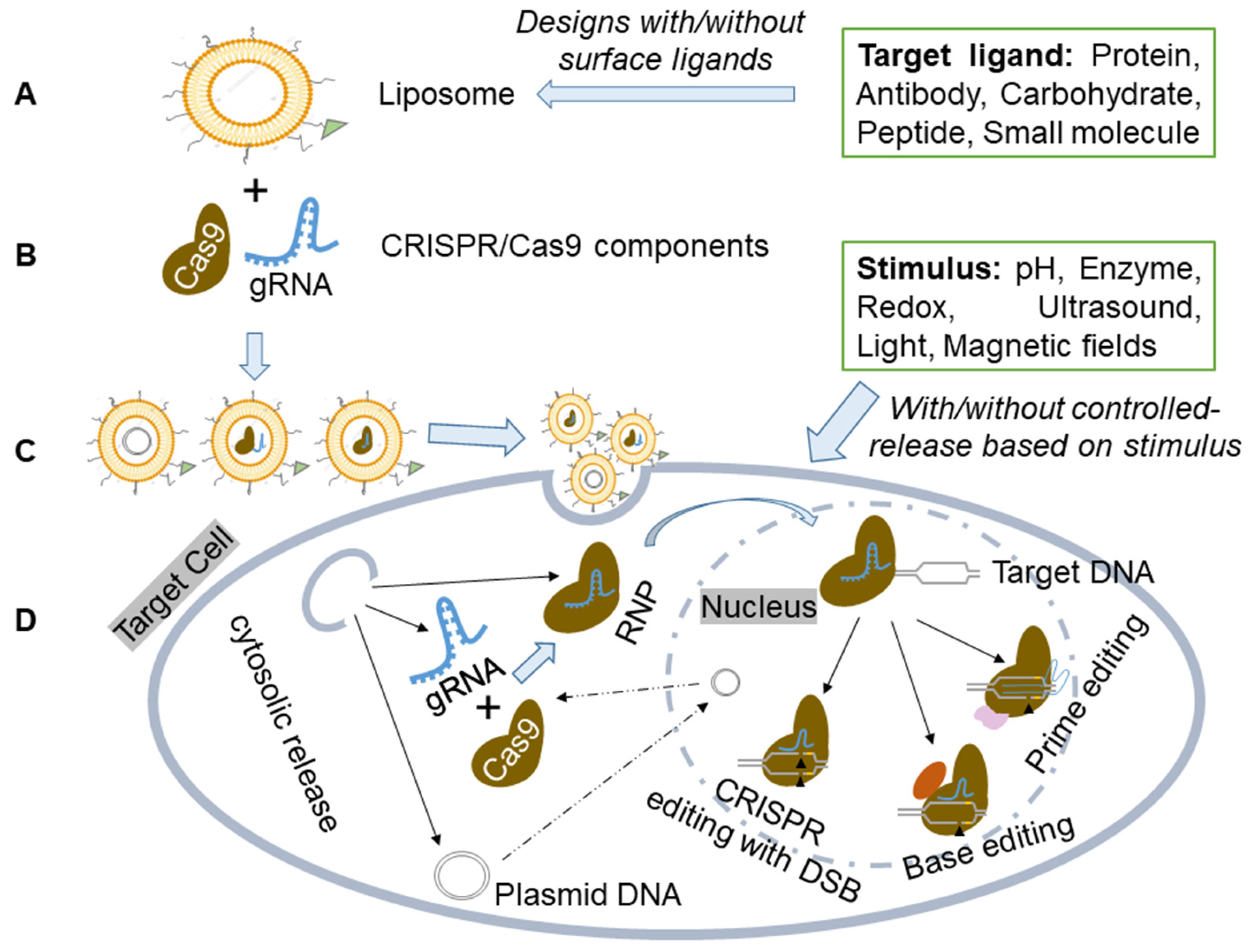
Figure 2. Schematic illustration of the liposome for CRISPR-based components delivery. (A) Liposome preparation. Liposome preparation includes various designs without/with surface ligands for specific binding. (B) Delivery of CRISPR/Cas9 components in plasmid, mRNA, or ribonucleoprotein (RNP) forms. (C) Liposome-based carriers to create a dependable and versatile delivery system for CRISPR/Cas9. (D) The liposome-based vector delivers CRISPR components to target cells or tissues, with/without controlled release based on specific stimuli sensitivity. The formed Cas9/gRNA RNP then enters the nucleus and interacts with target DNA, initiating genome editing through PAM and gRNA recognition of specific chromosomal DNA sequences. This enables precise genetic manipulation techniques like CRISPR with Double-Stranded Break (DSB), Base Editing, or Prime Editing.
Liposomes in CRISPR combination therapy carry additional agents like CRISPR-Cas9 components, chemotherapeutic drugs, siRNA, immunomodulatory, and gene regulatory components [13][36][37][40][51][57]. They possess the capability of being tailored with specific ligands, allowing for the targeted delivery of CRISPR components and other therapeutic agents to particular cells or tissues. This targeted approach enhances the overall effectiveness of treatment while minimizing the risk of off-target effects. The liposome-based CRISPR combination approach shows promise in treating diseases by combining CRISPR precision with synergistic effects.
Table 1 below summarizes the various liposome modifications and lipid compositions that have been used to specifically target and modulate gene expression both in vitro and in vivo.
Table 1. Summary of Various Studies in vitro and in vivo Using Different Liposome Modifications and Lipid Compositions.
| Formulation | Liposome Composition | Target Gene/Disease | Indication | Toxicity | Ref |
|---|---|---|---|---|---|
| MVL5-based Cationic liposomes | DOPC, DOPE, DOTAP, GMO, MVL5 | GFP | Knockdown in vitro |
considerable cytotoxicity compared to Lipofectamine 3000® | [13] |
| Cationic liposomes | DOTAP, DOPE, DSPE-PEG | IDUA/ Mucopolysaccha-ridosis type I |
Knock-in in vivo |
no obvious tissue toxicity and low cytotoxicity | [51] |
| Ultrasound-controlled cationic liposomes |
DLin-MC3-DMA, cholesterol, DSPC, DMG-PEG, DSPE-PEG | NFE2L2/Hepatocellular Carcinoma | Knockdown in vitro and in vivo |
no significant hepatorenal toxicity and low cytotoxicity | [40] |
| Ultrasound-activated microbubble liposome | lecithin, cholesterol, DPPC, DPPE | SRD5A2/ androgenic alopecia |
Knockdown in vitro and in vivo |
lower cytotoxicity | [37] |
| a liposome-coated mesoporous silica nanoparticle | DOPE, DOTAP, DSPE-PEG2000, cholesterol | pcsk9, apoc3, and angptl3/ hyperlipidemia |
Knockdown in vitro and in vivo |
N/A | [12] |
| lipid nanoparticle | DSPC, DOPE, DOPC, cholesterol, DMG-PEG | ANGPTL3/ hyperlipidemia |
Knockdown in vitro and in vivo |
minimal systemic toxicity | [36] |
| Peptide-modified liposome | Gal-PEG-DSPE, DOTAP, DOPE, Cholesterol | PCSK9/ hyperlipidemia |
Knockout in vitro and in vivo |
low cytotoxicity | [52] |
| Peptide-modified liposome | DOTAP, cholesterol, folate-PEG-succinyl-Cholesterol | DNMT1/ ovarian cancer |
Knockout in vitro and in vivo |
fewer cytotoxicity than paclitaxel | [50] |
| Peptide-modified liposome | tandem-peptide-lipid | GFP | Knockout in vitro |
significant toxicity with concentrations of peptides beyond 50× |
[57] |
| Peptide-modified liposome | DOTAP, cholesterol, DSPE-PEG2000-Maleimide | PLK-1/ brain cancer |
Knockout in vitro and in vivo |
lower cellular toxicity | [35] |
| Peptide-modified liposome | cKK-E12, DOPE, Cholesterol, C14-PEG | Pcsk9/ hyperlipidemia |
Knockout in vivo |
no induction of acute or chronic liver toxicity after gene editing |
[60] |
| Peptide-modified liposome | DOTAP, DOPE, DSPE-PEG, cholesterol | PLK1/ Melanoma |
Knockout in vitro and in vivo |
low cytotoxicity in vivo | [61] |
| pH-sensitive liposome |
DOTAP, DOPE, DSPE-PEG, Cholesterol | HPV16E6, E7/ cervical cancer |
Knockout in vitro and in vivo |
no significant toxicity in vivo | [41] |
| pH-sensitive liposome |
ECO | GFP | Knockout in vitro |
low cytotoxicity | [39] |
| Fusogenic liposomes | DSPE-PEG 2000, PC, cholesterol | PD-L1/ TNBC |
Knockout in vitro and in vivo |
low cytotoxicity | [38] |
| Multifunctional Liposomes | DOPE, DOTAP, Cholesterol, DSPE-PEG/DSPE-PEG-HA | MTH1/liver metastasis of NSCLC | Knockdown in vitro and in vivo |
lower cytotoxicity | [62] |
Abbreviation: DOPC: Dioleoylphosphocholine; DOPE Dioleoylphosphatidylethanolamine; GMO: Glycerol-monooleate; DOTAP: 2,3-Dioleyloxypropyltrimethylammonium chloride; MVL5: a pentavalent cationic lipid; D-Lin-MC3-DMA: 1,2-dilinoleoyl-3-dimethylaminopropane; GFP: Green fluorescence protein; IDUA: alpha-L-iduronidase; DSPC: 1,2-distearoyl-sn-glycero-3-phosphocholine; iduronidase; NFE2L2: Nuclear factor erythroid 2 (NFE2)-related factor 2; PEG: polyethylene glycol; DMG-PEG: Dimyristoylphosphatidylethanolamine-polyethylene glycol; PLK1: polo-like kinase 1; DNMT1: DNA methyltransferase 1; DPPC: phosphatidylcholines; DPPE: Diphosphatidylethanolamine; SRD5A2: 5alpha-reductase type 2; TNBC: triple-negative breast cancer; DSPE-PEG 2000: N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt; PC: phosphatidylcholine; Gal-PEG-DSPE: Galactose-polyethylene glycol-distearoylphosphatidylethanolamine; C14-PEG: Tetradecyl Polyethylene Glycol; cKK-E12: 3,6-bis [4-[bis(2-hydroxydodecyl)amino]butyl]-2,5-piperazinedione; Pcsk9: proprotein convertase subtilisin/kexin type 9; ECO: (1-aminoethyl)iminobis[N-(oleicylcysteinyl-1-amino-ethyl)propionamide]; Apo C3: apolipoprotein C3; ANGPTL3: Angiopoietin-like protein 3; HPV16E6 and E7: Human Papillomavirus E6 and E7; PDL1: programmed cell death ligand 1; MTH1: Human MutT homolog 1; NSCLC: non-small cell lung cancer, DSPE-PEG: distearoyl phosphoethanolamine-polyethylene glycol; and DSPE-PEG-HA: distearoyl phosphoethanolamine-polyethylene glycol-hyaluronic acid.
3.2. Various Types of Liposomes Used for CRISPR Delivery
Different types of liposomes have been developed for drug delivery. Each type has specific physiochemical characteristics that influence liposome stability, release kinetics, and interactions with target cells. Liposomes can be further modified with ligands to increase their specificity and efficiency. Summarized below are some of the commonly used liposomal delivery platforms.
3.2.1. Cationic Lipid-Based Liposomes
Cationic lipids, such as DOTAP and DLin-MC3-DMA, have been widely used for CRISPR/Cas9 delivery [13][40][51]. These liposomes can efficiently encapsulate and deliver the Cas9 protein and guide RNA into target cells. They form lipoplexes, which are complexes formed by the electrostatic interactions between cationic lipids and negatively charged CRISPR components. Cationic lipid-based liposomes have been shown to achieve efficient genome editing in various cell types and organisms. For example, Zhen et al. [58] investigated the effects of an RNA aptamer A10-liposome-CRISPR/Cas9 chimeras on cell-type binding specificity and remarkable gene silencing in vitro and on a significant regression of prostate cancer in vivo. The A10 aptamer specifically targeted prostate cancer cells expressing the cell-surface receptor PSMA (prostate-specific membrane antigen), allowing the delivery of therapeutic CRISPR/Cas9-gRNA targeting the survival gene polo-like kinase 1. The chimeras exhibited enhanced uptake by the target cells due to the modification with cationic liposomes, resulting in improved genome editing efficiency. The average particle size of the aptamer-liposome-CRISPR/Cas9 chimeras was approximately 150 nm. The toxicity of the chimeras was specific to the PLK1 CRISPR/Cas9 and correlated with differences in uptake by different delivery systems. Additionally, the liposome chimera preparations were less toxic than commercial lipofectamine-2000, and the toxicity increased somewhat with the addition of protamine and calf thymus DNA. In mice, A10-liposome-PLK1 CRISPR/Cas9 (40 μg A10-liposomes-gRNA + 40 μg Cas9) was IV injected. The CRISPR components were associated with the liposome surface.
3.2.2. Hybrid Liposomes
Hybrid liposomes are liposomes that incorporate other elements, such as mesoporous silica [12] or exosomes [38], to enhance their delivery efficiency. The specific composition of hybrid liposomes varies depending on the intended application. Liang et al. [63] developed a lipopolymer system combining lipids and polymers with the specific aptamer LC09 targeting Osteosarcoma (OS) cells. This system efficiently delivered CRISPR/Cas9 plasmids targeting the therapeutic gene VEGFA in OS. In a mouse model, LC09-modified lipopolymer complexes led to increased accumulation in tumors, resulting in enhanced genome editing, reduced malignancy, angiogenesis, and bone lesions compared to unmodified lipopolymer complexes. The PPC lipopolymer was composed of PEI, PEG, and CHOL. LC09-PPC-CRISPR/Cas9 was taken up via cellular mechanisms like macropinocytosis and caveolae-mediated endocytosis. In this study, CRISPR/Cas9 plasmids were encapsulated in PPC lipopolymer. LC09-PEG2000-DSPE, formed by conjugating LC09 with DSPE-PEG2000-Mal, was inserted into the surface of PPC, resulting in LC09-PPC-CRISPR/Cas9. The size of LC09-PPC-CRISPR/Cas9 was between 140–180 nm. The encapsulation efficiency of CRISPR/Cas9 was above 80%, and the loading efficiency of LC09 was about 85%. Both LC09 aptamer and CRISPR/Cas9 plasmid in PPC showed ideal serum stability, protecting them from serum enzyme degradations. They administered a CRISPR/Cas9 plasmid (0.75 mg kg−1) encapsulated in LC09-PPC vector intravenously to syngeneic orthotopic OS mice every two weeks for three cycles.
3.2.3. Fusogenic Liposomes
Fusogenic liposomes are designed to promote the efficient endosomal/lysosomal escape of the encapsulated CRISPR components [38]. These liposomes incorporate fusogenic peptides or pH-sensitive lipids that facilitate membrane fusion with endosomes, allowing the release of the liposome contents into the cytoplasm. Furthermore, Zhang et al. [52] developed Gal-LGCP (Gal-conjugated PEG-lipid/TAT-GNCs/Cas9/sgPcsk9) for targeted delivery of CRISPR/Cas9 components to edit the Pcsk9 gene, regulating plasma cholesterol levels via facilitating cellular uptake and lysosome escape. Gal-LGCP was formed by encapsulating TAT-GNCs/Cas9/sgPcsk9 in a lipid shell (DOTAP/DOPE) and modifying it with Gal-PEG-DSPE. Following intravenous injection, Gal-LGCP showed dose-dependent gene-editing efficacy (57% at 150 nM sgPcsk9) and lowered plasma LDL-C in mice by approximately 30%, comparable to those (36% to 52% down-regulation) in previous viral-based CRISPR studies [64]. Importantly, no off-target mutagenesis was detected. Gal-LGCP exhibits stable characteristics in different mediums, low cytotoxicity, about 60% Pcsk9-editing efficiency in vitro, and displays higher internalization efficiency than Lipofectamine2000/Cas9/sgPcsk9. The size of Gal-LGCP was approximately 100 nm.
3.2.4. PEGylated Liposomes
PEGylation, the attachment of polyethylene glycol (PEG) chains to liposome surfaces, can enhance the stability and circulation time of liposomes in the body [48][49]. PEGylated liposomes have been shown to enhance CRISPR genome editing efficiency, improve pharmacokinetics, and minimize immune responses [12][50][51][52][57][60][61]. For specific targeting, PEGylated liposomes can further be modified with ligands such as the Folate-a chemical ligand. He et al. [50] used a folate receptor-targeted liposome (F-LP) to deliver the CRISPR plasmid DNA co-expressing Cas9 and single-guide RNA targeting the ovarian cancer-related DNA methyltransferase 1 (DNMT1) gene (gDNMT1). By incorporating folate ligands, the complex can be selectively delivered to both paclitaxel-sensitive and -resistant ovarian cancer cells and inhibit tumor growth using CRISPR-based gene editing. In this study, PEGylated liposomes had a CRISPR/Cas9 component associated with PEG chains on the surface. The complex was safe and stable for intraperitoneal injection with fewer adverse effects than paclitaxel injection. Mice received intraperitoneal (i.p.) injections of the liposomal plasmid DNA (2.5 or 5 μg) dissolved in 200 μL glucose solution every 3 days for ten treatments. The particle size of F-LP/gDNMT1 was about 150 nm.
3.2.5. Multifunctional Liposomes
Multi-liposomes offer a more complex structure with multiple compartments for various applications and can be engineered to possess multiple functionalities, such as targeting, imaging, and therapeutic capabilities [62][65]. They can be equipped with targeting ligands, imaging agents, or other payloads to facilitate the delivery and tracking of CRISPR components in cells. For example, Wang et al. [62] developed a multifunctional non-viral vector that could actively target tumor cells and deliver Cas9/sgMTH1 plasmid (pMTH1) into cancer cell nuclei. This liposome was modified with distearoyl phosphoethanolamine-polyethylene glycol-hyaluronic acid (HA) to be endocytosed by HA-CD44 (cluster of differentiation protein 44) receptor interaction. It successfully inhibited the growth of non-small cell lung cancer by disrupting the MTH1 (Human MutT homolog 1) gene, promoting tumor tissue apoptosis, and reducing liver metastasis of non-small cell lung cancer (NSCLC) in mice. The active targeting ligand and nuclei-targeting component enabled efficient delivery of the cargo into cell nuclei, prolonging tumor-bearing mice survival. This modification enabled the potential of this multifunctional liposome for enhancing the efficacy of CRISPR/Cas9 genome editing in cancer therapy, utilizing its high loading capacity, safety, good serum stability, cellular uptake increase, and low immunogenicity. In this study, the CRISPR/Cas9 plasmids were effectively loaded into the “core” of the vector. PS@HA-Lip/pMTH1 (pMTH1: 1.5 μg/mL) was administered to A549 cells, whereas PS@HA-Lip/pMTH1 (plasmid dosage: 0.25 mg/kg) was intravenously injected into mice every 2 days, for a total of eight administrations.
3.2.6. Stimuli-Responsive Liposomes
Stimuli-responsive liposomes offer a versatile approach to controlled release, as they can be engineered to respond to various stimuli, including ultrasound, light, magnetic fields, or enzymes, to trigger drug, gene, or bioactive gas release at the target site [18][55][65][66][67][68][69]. Stimuli-responsive liposomes are currently being explored as a way to release the CRISPR components in response to ultrasound exposure [35][37][40] or pH changes [39][41]. Sun et al. [39] showed that new pH-sensitive amino lipids were designed and synthesized for intracellular delivery. These lipids formed stable and low cytotoxic nanoparticles with CRISPR/Cas9 DNA plasmids (100 to 200 nm in size). The amino lipid-DNA nanoparticles exhibited pH-sensitive hemolysis with minimal activity at pH 7.4 and increased hemolysis at acidic pH (pH = 5–6) via the mechanism of pH-sensitive amphiphilic endosomal escape and reductive cytosolic release. This study exhibited that the change in the head group did not affect the nanoparticle formation of the amino lipids and CRISPR/Cas9 plasmids with low cytotoxicity using the new amino lipids. The CRISPR/Cas9 components loaded into the cores of pH-sensitive liposomes were released in response to pH changes after cellular uptake. Cells were treated with the amino lipids at a dose of 1 μg/well and the nanoparticles at a dose of 2 μg/well in 12-well plates, respectively.
Recent studies have shown the feasibility and effectiveness of these liposome-based delivery systems in delivering CRISPR components to the desired site in diverse preclinical models. Various liposome systems have demonstrated successful outcomes for targeted gene editing strategies, with enhanced spatial and temporal control, thus paving the way for their potential translation into clinical applications. It is worth noting that different targeting methods can be combined to achieve a synergistic effect or tailor payload delivery for specific applications. The choice of targeting method depends on the disease type, target tissue or cells, and the desired therapeutic outcome.
Table 2 below provides an overview of various liposome-based CRISPR delivery platforms. Notably, advances in liposome-based CRISPR delivery technologies continue to address many of the shortcomings associated with each type of liposome.
Table 2. An overview of various liposome-based CRISPR delivery platforms.
| Liposome-Based CRISPR Delivery Platforms | Advantages | Disadvantages |
|---|---|---|
| Cationic Lipid-Based Liposomes |
|
|
| Hybrid Liposomes |
|
|
| Fusogenic Liposomes |
|
|
| PEGylated Liposomes |
|
|
| Multifunctional Liposomes |
|
|
| Stimuli-Responsive Liposomes |
|
|
This entry is adapted from the peer-reviewed paper 10.3390/ijms241612844
References
- Dever, D.P.; Bak, R.O.; Reinisch, A.; Camarena, J.; Washington, G.; Nicolas, C.E.; Pavel-Dinu, M.; Saxena, N.; Wilkens, A.B.; Mantri, S.; et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature 2016, 539, 384–389.
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260.
- Graham, C.; Hart, S. CRISPR/Cas9 gene editing therapies for cystic fibrosis. Expert Opin. Biol. Ther. 2021, 21, 767–780.
- Scott, A. How CRISPR is transforming drug discovery. Nature 2018, 555, S10–S11.
- Shi, J.; Wang, E.; Milazzo, J.P.; Wang, Z.; Kinney, J.B.; Vakoc, C.R. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat. Biotechnol. 2015, 33, 661–667.
- Taylor, D.W. The final cut: Cas9 editing. Nat. Struct. Mol. Biol. 2019, 26, 669–670.
- Wilkinson, R.A.; Martin, C.; Nemudryi, A.A.; Wiedenheft, B. CRISPR RNA-guided autonomous delivery of Cas9. Nat. Struct. Mol. Biol. 2019, 26, 14–24.
- Shen, S.; Sun, C.Y.; Du, X.J.; Li, H.J.; Liu, Y.; Xia, J.X.; Zhu, Y.H.; Wang, J. Co-delivery of platinum drug and siNotch1 with micelleplex for enhanced hepatocellular carcinoma therapy. Biomaterials 2015, 70, 71–83.
- Chen, P.J.; Liu, D.R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 2023, 24, 161–177.
- Raguram, A.; Banskota, S.; Liu, D.R. Therapeutic in vivo delivery of gene editing agents. Cell 2022, 185, 2806–2827.
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844.
- Gong, J.; Wang, H.X.; Lao, Y.H.; Hu, H.; Vatan, N.; Guo, J.; Ho, T.C.; Huang, D.; Li, M.; Shao, D.; et al. A Versatile Nonviral Delivery System for Multiplex Gene-Editing in the Liver. Adv. Mater. 2020, 32, e2003537.
- Sousa, D.A.; Gaspar, R.; Ferreira, C.J.O.; Baltazar, F.; Rodrigues, L.R.; Silva, B.F.B. In Vitro CRISPR/Cas9 Transfection and Gene-Editing Mediated by Multivalent Cationic Liposome-DNA Complexes. Pharmaceutics 2022, 14, 1087.
- Zhang, H.; Bahamondez-Canas, T.F.; Zhang, Y.; Leal, J.; Smyth, H.D.C. PEGylated Chitosan for Nonviral Aerosol and Mucosal Delivery of the CRISPR/Cas9 System in Vitro. Mol. Pharm. 2018, 15, 4814–4826.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387.
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12.
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22.
- Kee, P.H.; Moody, M.R.; Huang, S.L.; Kim, H.; Yin, X.; Peng, T.; Laing, S.T.; Klegerman, M.E.; Rahbar, M.H.; Vela, D.; et al. Stabilizing Peri-Stent Restenosis Using a Novel Therapeutic Carrier. JACC Basic Transl. Sci. 2020, 5, 1–11.
- Britton, G.L.; Kim, H.; Kee, P.H.; Aronowski, J.; Holland, C.K.; McPherson, D.D.; Huang, S.L. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation 2010, 122, 1578–1587.
- Klegerman, M.E.; Zou, Y.; Golunski, E.; Peng, T.; Huang, S.L.; McPherson, D.D. Use of thermodynamic coupling between antibody-antigen binding and phospholipid acyl chain phase transition energetics to predict immunoliposome targeting affinity. J. Liposome Res. 2014, 24, 216–222.
- Buchanan, K.D.; Huang, S.L.; Kim, H.; McPherson, D.D.; MacDonald, R.C. Encapsulation of NF-kappaB decoy oligonucleotides within echogenic liposomes and ultrasound-triggered release. J. Control Release 2010, 141, 193–198.
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359.
- Kohn, D.B.; Booth, C.; Kang, E.M.; Pai, S.Y.; Shaw, K.L.; Santilli, G.; Armant, M.; Buckland, K.F.; Choi, U.; De Ravin, S.S.; et al. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat. Med. 2020, 26, 200–206.
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668.
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417.
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081.
- Behr, J.P.; Demeneix, B.; Loeffler, J.P.; Perez-Mutul, J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc. Natl. Acad. Sci. USA 1989, 86, 6982–6986.
- Montier, T.; Benvegnu, T.; Jaffres, P.A.; Yaouanc, J.J.; Lehn, P. Progress in cationic lipid-mediated gene transfection: A series of bio-inspired lipids as an example. Curr. Gene Ther. 2008, 8, 296–312.
- Tros de Ilarduya, C.; Sun, Y.; Duzgunes, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170.
- Koltover, I.; Salditt, T.; Safinya, C.R. Phase diagram, stability, and overcharging of lamellar cationic lipid-DNA self-assembled complexes. Biophys. J. 1999, 77, 915–924.
- Wasungu, L.; Hoekstra, D. Cationic lipids, lipoplexes and intracellular delivery of genes. J. Control Release 2006, 116, 255–264.
- Behr, J.P. Gene transfer with synthetic cationic amphiphiles: Prospects for gene therapy. Bioconjugate Chem. 1994, 5, 382–389.
- Wheeler, C.J.; Felgner, P.L.; Tsai, Y.J.; Marshall, J.; Sukhu, L.; Doh, S.G.; Hartikka, J.; Nietupski, J.; Manthorpe, M.; Nichols, M.; et al. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proc. Natl. Acad. Sci. USA 1996, 93, 11454–11459.
- Budker, V.; Gurevich, V.; Hagstrom, J.E.; Bortzov, F.; Wolff, J.A. pH-sensitive, cationic liposomes: A new synthetic virus-like vector. Nat. Biotechnol. 1996, 14, 760–764.
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-Templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036.
- Qiu, M.; Glass, Z.; Chen, J.; Haas, M.; Jin, X.; Zhao, X.; Rui, X.; Ye, Z.; Li, Y.; Zhang, F.; et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. USA 2021, 118, e2020401118.
- Ryu, J.Y.; Won, E.J.; Lee, H.A.R.; Kim, J.H.; Hui, E.; Kim, H.P.; Yoon, T.J. Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy. Biomaterials 2020, 232, 119736.
- Sun, M.; Shi, W.; Wu, Y.; He, Z.; Sun, J.; Cai, S.; Luo, Q. Immunogenic Nanovesicle-Tandem-Augmented Chemoimmunotherapy via Efficient Cancer-Homing Delivery and Optimized Ordinal-Interval Regime. Adv. Sci. 2022, 10, e2205247.
- Sun, D.; Sun, Z.; Jiang, H.; Vaidya, A.M.; Xin, R.; Ayat, N.R.; Schilb, A.L.; Qiao, P.L.; Han, Z.; Naderi, A.; et al. Synthesis and Evaluation of pH-Sensitive Multifunctional Lipids for Efficient Delivery of CRISPR/Cas9 in Gene Editing. Bioconjugate Chem. 2019, 30, 667–678.
- Yin, H.; Sun, L.; Pu, Y.; Yu, J.; Feng, W.; Dong, C.; Zhou, B.; Du, D.; Zhang, Y.; Chen, Y.; et al. Ultrasound-Controlled CRISPR/Cas9 System Augments Sonodynamic Therapy of Hepatocellular Carcinoma. ACS Cent. Sci. 2021, 7, 2049–2062.
- Zhen, S.; Liu, Y.; Lu, J.; Tuo, X.; Yang, X.; Chen, H.; Chen, W.; Li, X. Human Papillomavirus Oncogene Manipulation Using Clustered Regularly Interspersed Short Palindromic Repeats/Cas9 Delivered by pH-Sensitive Cationic Liposomes. Hum. Gene Ther. 2020, 31, 309–324.
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851.
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087.
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 in Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Jubair, L.; Lam, A.K.; Fallaha, S.; McMillan, N.A.J. CRISPR/Cas9-loaded stealth liposomes effectively cleared established HPV16-driven tumours in syngeneic mice. PLoS ONE 2021, 16, e0223288.
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119.
- He, Z.Y.; Zhang, Y.G.; Yang, Y.H.; Ma, C.C.; Wang, P.; Du, W.; Li, L.; Xiang, R.; Song, X.R.; Zhao, X.; et al. In Vivo Ovarian Cancer Gene Therapy Using CRISPR-Cas9. Hum. Gene Ther. 2018, 29, 223–233.
- Schuh, R.S.; Poletto, E.; Pasqualim, G.; Tavares, A.M.V.; Meyer, F.S.; Gonzalez, E.A.; Giugliani, R.; Matte, U.; Teixeira, H.F.; Baldo, G. In vivo genome editing of mucopolysaccharidosis I mice using the CRISPR/Cas9 system. J. Control Release 2018, 288, 23–33.
- Zhang, L.; Wang, L.; Xie, Y.; Wang, P.; Deng, S.; Qin, A.; Zhang, J.; Yu, X.; Zheng, W.; Jiang, X. Triple-Targeting Delivery of CRISPR/Cas9 To Reduce the Risk of Cardiovascular Diseases. Angew. Chem. Int. Ed. Engl. 2019, 58, 12404–12408.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365.
- Huang, S.L. Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1167–1176.
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672.
- Jain, P.K.; Lo, J.H.; Rananaware, S.; Downing, M.; Panda, A.; Tai, M.; Raghavan, S.; Fleming, H.E.; Bhatia, S.N. Non-viral delivery of CRISPR/Cas9 complex using CRISPR-GPS nanocomplexes. Nanoscale 2019, 11, 21317–21323.
- Zhen, S.; Takahashi, Y.; Narita, S.; Yang, Y.C.; Li, X. Targeted delivery of CRISPR/Cas9 to prostate cancer by modified gRNA using a flexible aptamer-cationic liposome. Oncotarget 2017, 8, 9375–9387.
- Behr, M.; Zhou, J.; Xu, B.; Zhang, H. In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges. Acta Pharm. Sin. B 2021, 11, 2150–2171.
- Yin, H.; Song, C.Q.; Suresh, S.; Wu, Q.; Walsh, S.; Rhym, L.H.; Mintzer, E.; Bolukbasi, M.F.; Zhu, L.J.; Kauffman, K.; et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 2017, 35, 1179–1187.
- Wang, P.; Zhang, L.; Xie, Y.; Wang, N.; Tang, R.; Zheng, W.; Jiang, X. Genome Editing for Cancer Therapy: Delivery of Cas9 Protein/sgRNA Plasmid via a Gold Nanocluster/Lipid Core-Shell Nanocarrier. Adv. Sci. 2017, 4, 1700175.
- Wang, Y.; Tang, Y.; Zhao, X.M.; Huang, G.; Gong, J.H.; Yang, S.D.; Li, H.; Wan, W.J.; Jia, C.H.; Chen, G.; et al. A multifunctional non-viral vector for the delivery of MTH1-targeted CRISPR/Cas9 system for non-small cell lung cancer therapy. Acta Biomater. 2022, 153, 481–493.
- Liang, C.; Li, F.; Wang, L.; Zhang, Z.K.; Wang, C.; He, B.; Li, J.; Chen, Z.; Shaikh, A.B.; Liu, J.; et al. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials 2017, 147, 68–85.
- Rashid, S.; Curtis, D.E.; Garuti, R.; Anderson, N.N.; Bashmakov, Y.; Ho, Y.K.; Hammer, R.E.; Moon, Y.A.; Horton, J.D. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA 2005, 102, 5374–5379.
- Fan, J.; Liu, Y.; Liu, L.; Huang, Y.; Li, X.; Huang, W. A Multifunction Lipid-Based CRISPR-Cas13a Genetic Circuit Delivery System for Bladder Cancer Gene Therapy. ACS Synth. Biol. 2020, 9, 343–355.
- Huang, S.L.; Kee, P.H.; Kim, H.; Moody, M.R.; Chrzanowski, S.M.; Macdonald, R.C.; McPherson, D.D. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J. Am. Coll. Cardiol. 2009, 54, 652–659.
- Castelli, D.D.; Boffa, C.; Giustetto, P.; Terreno, E.; Aime, S. Design and testing of paramagnetic liposome-based CEST agents for MRI visualization of payload release on pH-induced and ultrasound stimulation. J. Biol. Inorg. Chem. 2014, 19, 207–214.
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884.
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013, 5, 96–107.
This entry is offline, you can click here to edit this entry!
