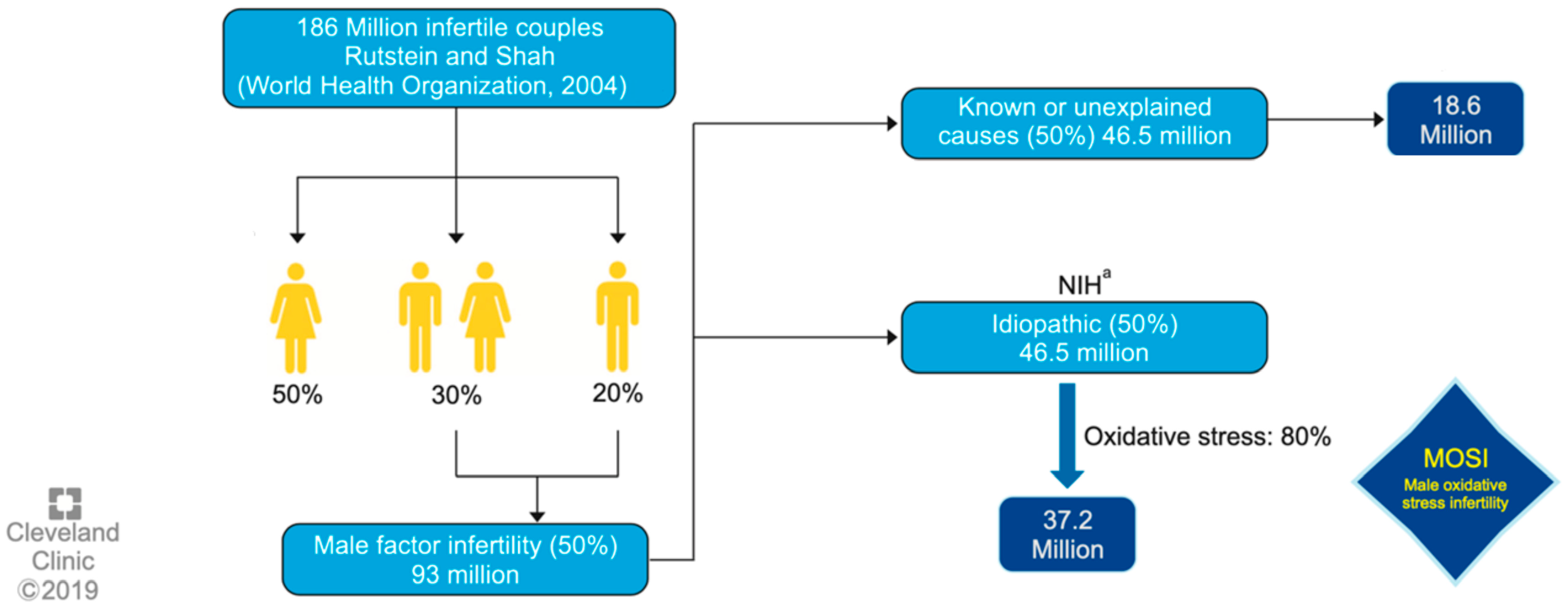
Male infertility (MI) involves various endogenous and exogenous facts. These include oxidative stress (OS), which is known to alter several physiological pathways and it is estimated to be present at high levels in up to 80% of infertile men. Infertility refers to the problem of couples who achieve a pregnancy but do not carry it to term. Male infertility (MI) is defined as the inability of a male to make a fertile female pregnant, also for a minimum of a one year of unprotected intercourse. As for the male factor, males are found to be solely responsible for 20–30% of infertility cases and contribute to 50% of cases overall.
- male infertility
- idiopathic
- oxidative stress
- reductive stress
1. Oxidative Stress and Idiopathic Male Infertility: MOSI
2. Antioxidants, as an Alternative in the Treatment of Idiopathic MI: MOXI
| Data Are Presented as the Number (%) or Median (Interquartile Range). | ||
|---|---|---|
| Antioxidants (n = 85) | Placebo (n = 86) | |
| Age (years) | 34.0 | 34.0 |
| (30.0, 37.0) | (30.0, 38.0) | |
| Body mass index (kg/m2) | 27.8 | 27.6 |
| (24.2, 31.7) | (24.4, 31.0) | |
| n = 82 | ||
| Ethnicity | ||
| Hispanic or Latino | 7 (8.2) | 5 (5.8) |
| Non-Hispanic | 72 (84.7) | 78 (90.7) |
| Unknown | 6 (7.1) | 3 (3.5) |
| Race | ||
| White | 63 (74.1) | 69 (80.2) |
| Black | 6 (7.1) | 7 (8.1) |
| Asian | 7 (8.2) | 2 (2.3) |
| American Indian or Alaska Native | 1 (12) | 1 (12) |
| Unknown | 8 (9.4) | 5 (5.8) |
| Mixed Race | 0 (0) | 2 (2.3) |
| Abnormal semen parameters | ||
| Single abnormal parameter | ||
| Sperm concentration ≤ 15 million/mL | 4 (4.7) | 5 (5.8) |
| Total motility ≤ 40% | 9 (10.6) | 10 (11.6) |
| Normal morphology # ≤ 4% | 33 (38.8) | 29 (33.7) |
| >1 abnormal parameters | 39 (45.9) | 42 (48.8) |
| Fathered a prior pregnancy ^ | ||
| Yes | 25 (29.4) | 38 (44.2) |
| No | 60 (70.6) | 48 (55.8) |
| Prior infertility treatment and/or surgery | ||
| Yes | 25 (29.4) | 24 (27.9) |
| No | 60 (70.6) | 62 (72.1) |
| Duration of infertility (months) | 24.0 | 24.0 |
| (18.0, 48.0) | (15.0, 36.0) | |
| n = 81 | n = 83 | |
| History of smoking | ||
| Never | 54 (63.5) | 47 (54.7) |
| Current | 8 (9.4) | 11 (12.8) |
| Former | 23 (27.1) | 28 (32.6) |
| History of alcohol use | ||
| Never | 6 (7.1) | 4 (4.7) |
| Current (in the past year) | 72 (84.7) | 81 (94.2) |
| Former (not in the past year) | 7 (8.2) | 1 (12) |
3. Other Antioxidants and Their Role as Biomarkers
This entry is adapted from the peer-reviewed paper 10.3390/antiox12081626
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37.
- Gunes, S.; Arslan, M.A.; Hekim, G.N.T.; Asci, R. The role of epigenetics in idiopathic male infertility. J. Assist. Reprod. Genet. 2016, 33, 553–569.
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17.
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive Oxygen Species and Sperm Function--In Sickness and In Health. J. Androl. 2012, 33, 1096–1106.
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052.
- Salonia, A.; Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Cazzaniga, W.; Chierigo, F.; Dehò, F.; Montanari, E.; Montorsi, F. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J. Androl. 2019, 21, 478–485.
- Lopes, F.; Pinto-Pinho, P.; Gaivão, I.; Martins-Bessa, A.; Gomes, Z.; Moutinho, O.; Oliveira, M.M.; Peixoto, F.; Pinto-Leite, R. Sperm DNA damage and seminal antioxidant activity in subfertile men. Andrologia 2021, 53, e14027.
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reprod. Med. Biol. 2020, 20, 41–52.
- Symeonidis, E.N.; Evgeni, E.; Palapelas, V.; Koumasi, D.; Pyrgidis, N.; Sokolakis, I.; Hatzichristodoulou, G.; Tsiampali, C.; Mykoniatis, I.; Zachariou, A.; et al. Redox Balance in Male Infertility: Excellence through Moderation—“Μέτρον ἄριστον”. Antioxidants 2021, 10, 1534.
- Agarwal, A.; Parekh, N.; Selvam, M.K.P.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312.
- Micoogullari, U.; Cakici, M.C.; Kilic, F.U.; Kisa, E.; Caglayan, A.; Neselioglu, S.; Karatas, O.F.; Erel, O. Evaluation of the role of thiol/disulfide homeostasis in the etiology of idiopathic male infertility with a novel and automated assay. Syst. Biol. Reprod. Med. 2022, 68, 162–168.
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014, CD007411.
- Dutta, S.; Sengupta, P.; Roychoudhury, S.; Chakravarthi, S.; Wang, C.W.; Slama, P. Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation. Antioxidants 2022, 11, 167.
- Chen, G.; Kathrins, M.; Ohlander, S.; Niederberger, C. Medical management of male infertility: Now and future. Curr. Opin. Urol. 2023, 33, 10–15.
- Adewoyin, M.; Ibrahim, M.; Roszaman, R.; Isa, M.L.M.; Alewi, N.A.M.; Rafa, A.A.A.; Anuar, M.N.N. Male Infertility: The Effect of Natural Antioxidants and Phytocompounds on Seminal Oxidative Stress. Diseases 2017, 5, 9.
- Agarwal, A.; Sekhon, L.H. The role of antioxidant therapy in the treatment of male infertility. Hum. Fertil. 2010, 13, 217–225.
- Steiner, A.Z.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Usadi, R.; Baker, V.L.; Coward, R.M.; et al. The effect of antioxidants on male factor infertility: The Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertil. Steril. 2020, 113, 552–560.e3.
- Knudtson, J.F.; Sun, F.; Coward, R.M.; Hansen, K.R.; Barnhart, K.T.; Smith, J.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Zhang, H.; et al. The relationship of plasma antioxidant levels to semen parameters: The Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. J. Assist. Reprod. Genet. 2021, 38, 3005–3013.
- Agarwal, A.; Cannarella, R.; Saleh, R.; Harraz, A.M.; Kandil, H.; Salvio, G.; Boitrelle, F.; Kuroda, S.; Farkouh, A.; Rambhatla, A.; et al. Impact of Antioxidant Therapy on Natural Pregnancy Outcomes and Semen Parameters in Infertile Men: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. World J. Men’s Health 2023, 41, 14–48.
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113.
- Sengupta, P.; Agarwal, A.; Pogrebetskaya, M.; Roychoudhury, S.; Durairajanayagam, D.; Henkel, R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod. Biomed. Online 2018, 36, 311–326.
- Lucignani, G.; Jannello, L.M.I.; Fulgheri, I.; Silvani, C.; Turetti, M.; Gadda, F.; Viganò, P.; Somigliana, E.; Montanari, E.; Boeri, L. Coenzyme Q10 and Melatonin for the Treatment of Male Infertility: A Narrative Review. Nutrients 2022, 14, 4585.
- Alahmar, A.T.; Sengupta, P.; Dutta, S.; Calogero, A.E. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin. Exp. Reprod. Med. 2021, 48, 150–155.
- Alahmar, A.T.; Naemi, R. Predictors of pregnancy and time to pregnancy in infertile men with idiopathic oligoasthenospermia pre- and post-coenzyme Q10 therapy. Andrologia 2022, 54, e14385.
- Balercia, G.; Mancini, A.; Paggi, F.; Tiano, L.; Pontecorvi, A.; Boscaro, M.; Lenzi, A.; Littarru, G.P. Coenzyme Q10 and male infertility. J. Endocrinol. Investig. 2009, 32, 626–632.
- Hu, K.L.; Ye, X.; Wang, S.; Zhang, D. Melatonin Application in Assisted Reproductive Technology: A Systematic Review and Meta-Analysis of Randomized Trials. Front. Endocrinol. 2020, 11, 160.
- Xing, S.; Guo, Z.; Lang, J.; Zhou, M.; Cao, J.; He, H.; Yu, L.; Zhou, Y. N-Acetyl-l-cysteine ameliorates gestational diabetes mellitus by inhibiting oxidative stress. Gynecol. Endocrinol. 2023, 39, 2189969.
- Khaw, S.C.; Wong, Z.Z.; Anderson, R.; da Silva, S.M. l-carnitine and l-acetylcarnitine supplementation for idiopathic male infertility. Reprod. Fertil. 2020, 1, 67–81.
- Zhou, Z.; Cui, Y.; Zhang, X.; Zhang, Y. The role of N-acetyl-cysteine (NAC) orally daily on the sperm parameters and serum hormones in idiopathic infertile men: A systematic review and meta-analysis of randomised controlled trials. Andrologia 2021, 53, e13953.
- Wei, G.; Zhou, Z.; Cui, Y.; Huang, Y.; Wan, Z.; Che, X.; Chai, Y.; Zhang, Y. A Meta-Analysis of the Efficacy of L-Carnitine/L-Acetyl-Carnitine or N-Acetyl-Cysteine in Men With Idiopathic Asthenozoospermia. Am. J. Men’s Health 2021, 15, 15579883211011371.
- Tenório, M.C.d.S.; Graciliano, N.G.; Moura, F.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967.
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982.
- Gvozdjáková, A.; Kucharská, J.; Dubravicky, J.; Mojto, V.; Singh, R.B. Coenzyme Q10,α-Tocopherol, and Oxidative Stress Could Be Important Metabolic Biomarkers of Male Infertility. Dis. Markers 2015, 2015, 827941.
- Banks, N.; Sun, F.; Krawetz, S.A.; Coward, R.M.; Masson, P.; Smith, J.F.; Trussell, J.; Santoro, N.; Zhang, H.; Steiner, A.Z. Male vitamin D status and male factor infertility. Fertil. Steril. 2021, 116, 973–979.
- Bosdou, J.K.; Konstantinidou, E.; Anagnostis, P.; Kolibianakis, E.M.; Goulis, D.G. Vitamin D and Obesity: Two Interacting Players in the Field of Infertility. Nutrients 2019, 11, 1455.
- Sidhom, K.; Panchendrabose, K.; Mann, U.; Patel, P. An update on male infertility and intratesticular testosterone—Insight into novel serum biomarkers. Int. J. Impot. Res. 2022, 34, 673–678.
- Llavanera, M.; Delgado-Bermúdez, A.; Ribas-Maynou, J.; Salas-Huetos, A.; Yeste, M. A systematic review identifying fertility biomarkers in semen: A clinical approach through Omics to diagnose male infertility. Fertil. Steril. 2022, 118, 291–313.
- Preianò, M.; Correnti, S.; Butt, T.A.; Viglietto, G.; Savino, R.; Terracciano, R. Mass Spectrometry-Based Untargeted Approaches to Reveal Diagnostic Signatures of Male Infertility in Seminal Plasma: A New Laboratory Perspective for the Clinical Management of Infertility? Int. J. Mol. Sci. 2023, 24, 4429.
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433.
