Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
MicroRNAs (miRNAs) are small non-coding RNA molecules that are 18–25 nucleotides long (22 nucleotides on average) and involved in the transcriptional and post-transcriptional regulation of gene expression by RNA interference, which is of great interest to molecular biologists, geneticists, and biochemists. These molecules are mainly present intracellularly, but there is also an extracellular (circulating) microRNA fraction. The existence and functions of more than 2500 human miRNAs are known.
- miRNA
- Alzheimer’s disease
- ischemia–reperfusion injury
- blood–brain barrier
- RNA biology
- neuroinflammation
1. The Role of MicroRNAs in Signaling Pathways of Alzheimer’s-Type Neurodegeneration
Disruption of epigenetic regulation and alterations in microRNA expression, which usually occur in conjunction, are important factors in the pathogenesis of many neurological disorders [8,9].
Several recent and extensive systematic reviews [68,69,70,71,72,73] have addressed the role of microRNAs in the pathogenesis of Alzheimer’s disease. These reviews explore numerous microRNA variants involved in the epigenetic regulation of the neurodegenerative process that serve as potential targets for diagnosis and targeted therapy.
Examples of such microRNAs include the molecules miR-200b, miR-135a, miR-10a-5p, miR-142a-5p, miR-146a-5p, miR-155-5p, miR-211-5p, miR-455-5p, miR-34a, miR-125b, miR-181c, miR-9, miR-191-5p, miR-181c, and miR-206, which are considered potential diagnostic markers for Alzheimer’s disease [74]. Additionally, attention should be paid to microRNA molecules that possess both diagnostic and therapeutic potential: miR-128, miR-574, miR-146-a, miR-181, miR-132, miR-188-5p, and miR-137 [74].
There are also studies describing modern bioinformatic approaches that use artificial intelligence and machine learning algorithms for identifying new biomarkers and improving the accuracy of molecular diagnosis of Alzheimer’s disease [75,76,77,78,79].
Some review papers present important data on other non-coding RNAs, such as circular RNAs and long non-coding RNAs, which participate in the pathogenesis of neurodegeneration and regulate the interplay between microRNAs/mRNAs/regulatory signaling pathways, which are mediated genetically [80,81]. These RNA molecules are often considered by researchers as more efficient targets for diagnostic and therapeutic strategies. Another important contemporary research direction in Alzheimer’s disease pathogenesis is the study of the regulatory functions of both microRNAs localized in specific organelles (such as mitochondria [82]) and exosomal microRNAs [83,84].
The assessment of neurovascular dysfunction is of particular interest as it plays a crucial role in the onset and progression of the neurodegenerative process associated with the accumulation of β-amyloid peptide in brain neurons and cerebral vessel walls [85,86]. Special attention is given to the dysfunction of the blood–brain barrier, reduced cerebral blood flow, and impaired vascular clearance of beta-amyloid from the brain into the glymphatic system and meningeal lymphatic vessels [87]. These disturbances may, in turn, be linked to the reprogramming of epigenetic regulation [88,89].
According to several authors, RNA interference mediated by microRNAs can initiate neurovascular events leading to Alzheimer’s-type neurodegeneration [90,91,92,93]. This provides a strong theoretical basis for the development of new directions in targeted genetic therapy for neurodegenerative diseases [94,95,96,97].
2. Regulation of Signaling Pathways in Ischemia and Reperfusion Injury of Nerve Cells Involving microRNAs
In in vitro models, it has been shown that the activation of miR-496 and miR-874-3p reduces the consequences of ischemic–reperfusion injury in neurons by negatively regulating BCL2L14 and BMF/BCL2L13, respectively [98,99]. Activation of miR-92b-3p expression inhibits apoptosis, mitochondrial dysfunction, and inflammation through the inhibition of TRAF3 [100]. MiR-182-5p and miR-193b-3p also exert neuroprotective effects in cerebral ischemia–reperfusion injury by negatively regulating the inflammatory response mediated by Toll-like receptor 4 and 5-lipoxygenase, respectively [101,102]. Additionally, miR-30c acts by inhibiting neuronal apoptosis in ischemia–reperfusion injury of the brain, thus suppressing the expression of SOX9/MAPK [103,104], while miR-449a downregulates the expression of amphiregulin (AREG) [105].
Similar effects are observed with the inhibition of miR-19a-3p, which reduces the extent and area of cerebral ischemia–reperfusion injury by regulating inflammation and apoptosis through increased expression of IGFBP3 both in vivo and in vitro [106]. It has also been discovered that inhibiting exosomal miR-200a-3p/141-3p, which originates from astrocytes and targets the gene SIRT1 and its associated signaling pathways, reduces the pathological consequences of cerebral ischemia–reperfusion injury in a mouse model [107]. MiR-370 has been shown to exacerbate neuronal reperfusion injury by impacting the expression of SIRT6 and the regulation mechanism of the Nrf2/ARE signaling pathway [108], while the exosomal form of this microRNA (370-3p) increases blood–brain barrier permeability during ischemia–reperfusion injury through the interference of MAPK1 [109].
Long non-coding RNA MEG3, by binding to miR-485, promotes the exacerbation of cerebral ischemia–reperfusion injury through potentiating pyroptosis via AIM2 [110]. Additionally, the knockdown of miR-155-5p acts by inhibiting the pyroptosis mechanism and inflammation through interference with DUSP14 [111,112]. It should be noted that Zhang L and colleagues [113], without emphasizing the orientation of miR-155, also demonstrated its activating influence on apoptosis and inflammatory processes in neural tissue.
In addition to the mentioned studies, there are several other works concerning the role of microRNAs in the pathogenesis of ischemia–reperfusion injury and the signaling pathways through which their effects are realized during RNA interference (Figure 1). The majority of neuroprotective mechanisms affected by the overexpression or inhibition of microRNAs involve anti-apoptotic, anti-inflammatory, and antioxidant signaling pathways.
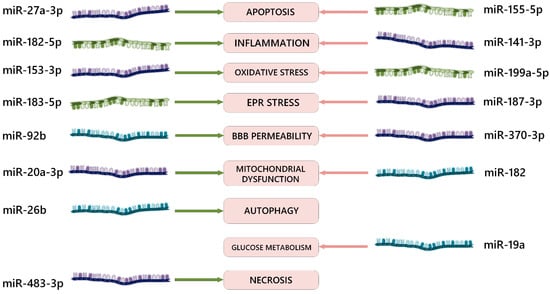
Figure 1. Examples of effects of various miRNAs on signaling pathways in the pathogenesis of ischemia–reperfusion brain injury. EPR = endoplasmic reticulum; BBB = blood–brain barrier; green arrows = positive biological effect of RNA interference; red arrows = negative biological effect of RNA interference. The dark blue color of the molecules indicates microRNA-3p (sense); the olive color indicates microRNA-5p (antisense); the aquamarine color indicates microRNAs with an unknown formation end from pre-microRNA.
Most studies from various researchers have demonstrated unidirectional effectiveness regarding the neuroprotective role of miR-10b-3p [116,117], miR-124-3p [119,120], miR-141-3p [107,131], miR-153-3p [141,142], miR-182-5p [101,150], miR-20a-3p [162,163], miR-22-3p [171,172], miR-24-3p [175,176], miR-27a-3p [181,182,183], miR-342-5p [191,192], miR-455-3p [200,201], miR-488-3p [203,204], and miR-92b-3p [100,221] as well as the damaging role of miR-141-3p [107,131], miR-155-5p [111,112], and miR-182 [148,149]. Therefore, these specific microRNAs should be considered as priority targets for further translation into clinical practice.
Indeed, along with the discovery of unidirectional effects of microRNA expression, contradictory results concerning the same molecules are also encountered. For example, according to the findings presented by Jia T et al. [205], activation of miR-489-3p expression in in vivo and in vitro models reduces the pathological consequences of cerebral ischemia–reperfusion by inhibiting histone deacetylase 2 (HDAC2), thereby reducing apoptosis intensity and enhancing cell viability. In contrast, Song L et al. [206] obtained opposing results: in mice subjected to temporary middle cerebral artery occlusion, an intensification of oxidative stress and neuron apoptosis was observed under conditions of increased miR-489-3p levels, which inhibits Sirtuin1 (SIRT1).
In the study investigating the effects of miR-421-3p in cerebral ischemia–reperfusion [199], both in vivo and in vitro models showed that increased expression of miR-421-3p reduces the intensity of inflammation through the YTHDF1/NF-κB p65 signaling pathway. On the contrary, Xu J. et al. [198] found a reverse effect of this microRNA concerning the intensity of apoptosis and inflammation in models of ischemia–reperfusion nerve tissue damage, mediated through the myocyte enhancer factor 2C (MEF2C).
In the study by Wei XY et al. [170], positive effects of the long non-coding RNA (lncRNA) RPL34-AS1 were observed in patients with stroke as well as in cerebral ischemia in rats and in an OGD cell model. This molecule inhibits miR-223-3p, which targets the insulin-like growth factor 1 receptor (IGF1R). The authors attribute the positive effects of RPL34-AS1 to its influence on this specific mechanism. However, there are contradictory results regarding the effects of miR-223-3p in studies focusing on the circular RNA circPDS5B and its impact on angiogenesis [168] and regarding the positive impact of miR-223-3p expression on the development of the inflammatory response in the zone of ischemia–reperfusion and its surrounding area [169].
The evaluation of the effects of miR-19a and its sense form miR-19a-3p shows a negative role in the mechanism of cerebral ischemia–reperfusion injury played by excessive stimulation of apoptosis and inflammation [106,156,157]. Similar observations are confirmed for the structurally related miR-19b-3p, which also intensifies the neuroinflammatory process in the ischemia–reperfusion zone [157]. However, these observations are contradicted by data showing that knockdown of the long non-coding RNA H19 and overexpression of miR-19a-3p attenuated the severity of ischemia–reperfusion-induced oxidative stress and apoptosis in neurons, as reported by Gao N. et al. [158].
The study of the effects of the lncRNA Malat1 revealed a positive influence of miR-26b [179] and a negative impact of miR-142-3p expression [132] on apoptosis and cell proliferation during experimental brain hypoxia–ischemia. However, the results from Li J. et al. [133] show an opposite effect when miR-142-3p expression is enhanced: inhibiting FBXO3. It is important to note that this study has limitations as it was conducted only on an in vitro model.
In two studies investigating the role of miR-140-3p on in vitro models with OGD, opposite effects were demonstrated: on the PC12 cell line, an enhancement of miR-140-3p expression showed a weakening effect on apoptosis and oxidative stress [130], while on the N2a cell line, an overexpression of miR-140-3p potentiated apoptosis and oxidative stress [129]. Supporting the greater significance of the first study and the neuroprotective role of this molecule is the fact that the in vitro results obtained by Zhang Y. et al. were replicated in the study on patients with ischemic stroke.
Another contradiction in determining the role of miRNAs in the pathogenesis of ischemia–reperfusion brain injury is the data on miR-128-3p. They indicate its proapoptotic efficacy in in vitro and in vivo models by inhibiting the FOXO/Relaxin signaling pathway [122] as well as the neuroprotective efficacy (potentiation of differentiation and myelination processes) of this miRNA in exosomal localization in an in vivo experiment [123].
Moreover, the studies mentioned above demonstrate that miR-135a, miR-181c, and miR-211-5p, whose expression plays a positive role in protection against cerebral ischemia–reperfusion injury, also act as neuroprotective agents in Alzheimer’s disease [74]. Conversely, the overexpression of miR-155-5p, miR-200a-3p, and miR-206 leads to damage to neural tissue both in the context of ischemia–reperfusion and Alzheimer’s-like neurodegeneration [74].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241612899
This entry is offline, you can click here to edit this entry!
