Coenzyme A (CoA) is synthetized from pantothenic acid (commonly known as vitamin B5). The importance of CoA as a carrier of acyl residues in cell metabolism is well understood. Coenzyme A participates in more than 100 different catabolic and anabolic reactions, including those involved in the metabolism of lipids, carbohydrates, proteins, ethanol, bile acids, and xenobiotics.
- Coenzyme A
- cell metabolism
- Circulating Lipid-Lowering Supplemental Agents
1. Introduction
Coenzyme A (CoA or CoA-SH) is an essential cofactor of cellular metabolism in all living organisms. Pantothenic acid (Pan, commonly known as vitamin B5) is the only nutritionally essential component involved in the synthesis of CoA, which is required for many biochemical processes (see below) and for the synthesis of an acyl carrier protein that is involved in fatty acid biosynthesis [1][2]. Moreover, Pan triggers immune cells to produce cytokines [3].
The name of Pan is derived from the root word pantos, which means “everywhere.” The widespread availability of Pan in the diet (both in products of animal, including milk, and plant origin) means that, in humans, Pan deficiency occurs largely due to severe malnutrition with combined vitamin deficiencies. However, it was recently found that cerebral Pan levels are significantly decreased compared to the control values in patients with Huntington’s disease [4]. Moreover, Pan deficiency results in greying hair in rats and other animals. Studies have shown that vitamin B5 supplementation with calcium pantothenate can promote grey hair darkening [5]. Neither the toxicity nor the upper intake level have been established [6]. However, in some individuals consuming very large doses of Pan supplements (approximately 10 g per day), gastrointestinal distress and diarrhea have been observed [2].
The unique chemical structure of CoA-SH allows it to be used to activate carboxylic acids involved in both catabolic and anabolic reactions. Generally, in humans, CoA-SH is required for (a) chemical reactions that generate energy from fat, carbohydrates, protein and catabolism of ethanol; (b) biosynthesis of fatty acids (necessary for biosynthesis of: triacylglycerols, phospholipids, sphingolipids), cholesterol, acetylcholine, prenyl moieties, bile acids, ketone bodies, heme, melatonin, glycosaminoglycans, glycoproteins, gangliosides, proteoglycans, and others) [7][8]; (c) regulation of metabolism (direct allosteric regulation of pyruvate dehydrogenase kinase-PDK, carnitine palmitoyltransferase 1—CPT1 and indirect regulation of carbamoyl phosphate syntethase I); and (d) gene expression (e.g., histone acetylation) [9]. Moreover, CoA-SH and its thioester derivatives (mainly acetyl-CoA and benzoyl-CoA) participate in detoxification reactions during which compounds are formed and then excreted in urine, e.g., hippuric or mercapturic acids [10]. The recently discovered, unconventional function of free CoA (CoA-SH) is protein CoAlation. This process is related to redox regulation and antioxidant defense [11]. Exemplary reactions that involve CoA-SH as a substrate and reactions in which CoA-SH is released as a product in human cells are presented in Table 1 and Table 2.
Table 1. Examples of reactions with the participation of CoA-SH as a substrate in human cells; based on Ridgway and Mcleod and the UniProt database [7][12].
| CoA-SH as a Substrate | |||
|---|---|---|---|
| Enzyme | Reaction | Process | |
| Lipid metabolism | acyl-CoA synthetases (ACS) | fatty acid + CoA-SH + ATP → fatty acyl-CoA + AMP + PPi | fatty acids activation |
| carnitine palmitoyltransferase 2 (CPT2) | acylcarnitine + CoA-SH → carnitine + fatty acyl-CoA | carnitine shuttle | |
| thiolases e.g., β-ketoacyl-CoA thiolase |
acyl-CoA + CoA-SH → acyl(n carbon-2)-CoA + acetyl-CoA acetoacetyl-CoA + CoA-SH → 2 acetyl-CoA |
fatty acids oxidation ketone bodies oxidation |
|
| ATP-citrate lyase (ACLY) | citrate + ATP + CoA-SH → oxaloacetate + acetyl-CoA + ADP + Pi | lipogenesis, synthesis of cholesterol and others | |
| Carbohydrate metabolism | pyruvate dehydrogenase complex (PDC) | pyruvate + CoA-SH + NAD+ → acetyl-CoA + NADH + H+ + CO2 | oxidative decarboxylation of pyruvate |
| Amino acids metabolism | branched-chain α-keto acid dehydrogenase complex | α-ketoisovaleric acid + CoA-SH + NAD+ → isobutyryl-CoA + NADH + H+ + CO2 α-ketoisocapronic acid + CoA-SH + NAD+ → iso-valeryl-CoA + NADH + H+ + CO2 α-keto-β-methylvaleric acid + CoA-SH + NAD+ → α-methylbutyryl-CoA + NADH + H+ + CO2 |
oxidative decarboxylation of branched-chain α-keto acids |
| Lipid, carbohydrate, amino acids and ethanol metabolism | α-oxoglutarate dehydrogenase complex | α-oxoglutarate + CoA-SH + NAD+ → succinyl-CoA + NADH + H+ + CO2 | tricarboxylic acid cycle |
| acetyl-CoA synthetase | acetate + CoA-SH +ATP → acetyl-CoA +AMP + PPi | ethanol metabolism, acetate formed by gut microbiota metabolism | |
Table 2. Examples of reactions involving CoA-SH as a product in human cells; based on Ridgway and Mcleod and the UniProt database [7][12].
| CoA-SH as a Product | |||
|---|---|---|---|
| Enzyme | Reaction | Process | |
| Lipid metabolism | fatty acid synthase (FASN) | 7 malonyl-CoA + acetyl-CoA + 14 NADPH + 14 H+ → palmitate + 14 NADP+ + 7 CO2 + 6 H2O + 8 CoA-SH | lipogenesis |
| fatty acid elongases (ELOVLs) | fatty acyl-CoA + malonyl-CoA → β-keto-acyl-CoA + CO2 + CoA-SH or fatty acyl-CoA + acetyl-CoA → β-keto-acyl-CoA + CoA-SH |
microsomal elongation of fatty acid chains mitochondrial elongation of fatty acid chains |
|
| acyltransferases e.g., diacylglycerol O-acyltransferase (DGAT) e.g., acyl-CoA:cholesterol acyltransferase (ACAT) |
1,2-diacylglycerol + fatty acyl-CoA → triacylglycerol + CoA-SH cholesterol + acyl-CoA → cholesteryl ester + CoA-SH |
triacylglycerol synthesis cholesterol metabolism |
|
| carnitine palmitoyltransferase 1 (CPT1) | carnitine + acyl-CoA → acylcarnitine + CoA-SH | carnitine shuttle | |
| 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) | HMG-CoA + 2 NADPH + 2 H+ → mevalonate +2 NADP+ + CoA-SH | synthesis of cholesterol, cholecalciferol (skin), prenyl moieties | |
| acyl-CoA thioesterases | fatty acyl-CoA + H2O → free fatty acid + CoA-SH | regulation of intracellular levels of acyl-CoA, free fatty acids and CoASH | |
| Lipid, carbohydrate, amino acids and ethanol metabolism | citrate synthase | acetyl-CoA + oxaloacetate + H2O → citrate + CoA-SH | tricarboxylic acid cycle |
| succinate thiokinase (also called succinyl-CoA synthetase) |
succinyl-CoA + ADP (GDP) + Pi → succinate + ATP (GTP) + CoA-SH | tricarboxylic acid cycle | |
| Others | acetyltransferases e.g., choline O-acetyltransferase e.g., histone acetyltransferase (HAT) |
choline + acetyl-CoA → acetylcholine + CoA-SH histone-Lys + acetyl-CoA→ histone-Lys-acetyl + CoA-SH |
neurotransmitters synthesis protein acetylation |
The major pools of CoA-SH and its thioesters are found in mitochondria and the cytosol. Other organelles (peroxisome, nuclei, lysosomes, and endoplasmic reticulum) contain much less CoA-SH. In mitochondria, CoA-SH is used in: (a) fatty acids and ketone bodies oxidation (as a substrate for thiolases and carnitine palmitoyltransferase 2—CPT2); (b) tricarboxylic acid cycle (as a substrate for α-oxoglutarate dehydrogenase); and (c) oxidative decarboxylation of pyruvate and branched-chain α-keto acids [13]. In the cytosol, CoA-SH is mainly used in reactions catalyzed by ATP-citrate lyase (ACLY) and acyl-CoA synthetase (ACS) (Table 1).
The acyl groups formed during the metabolism of glucose, amino acids and fatty acids in human cells and those produced by gut microbiota are attached to CoA-SH to form its thioester derivatives, such as acetyl-CoA, succinyl-CoA, propionyl-CoA, isovaleryl-CoA, isobutyryl-CoA, α-methylbutyryl-CoA, and fatty acyl-CoA (commonly referred to as acyl-CoA), e.g., palmitoyl-, oleoyl-, and stearoyl-CoA (Figure 1A).
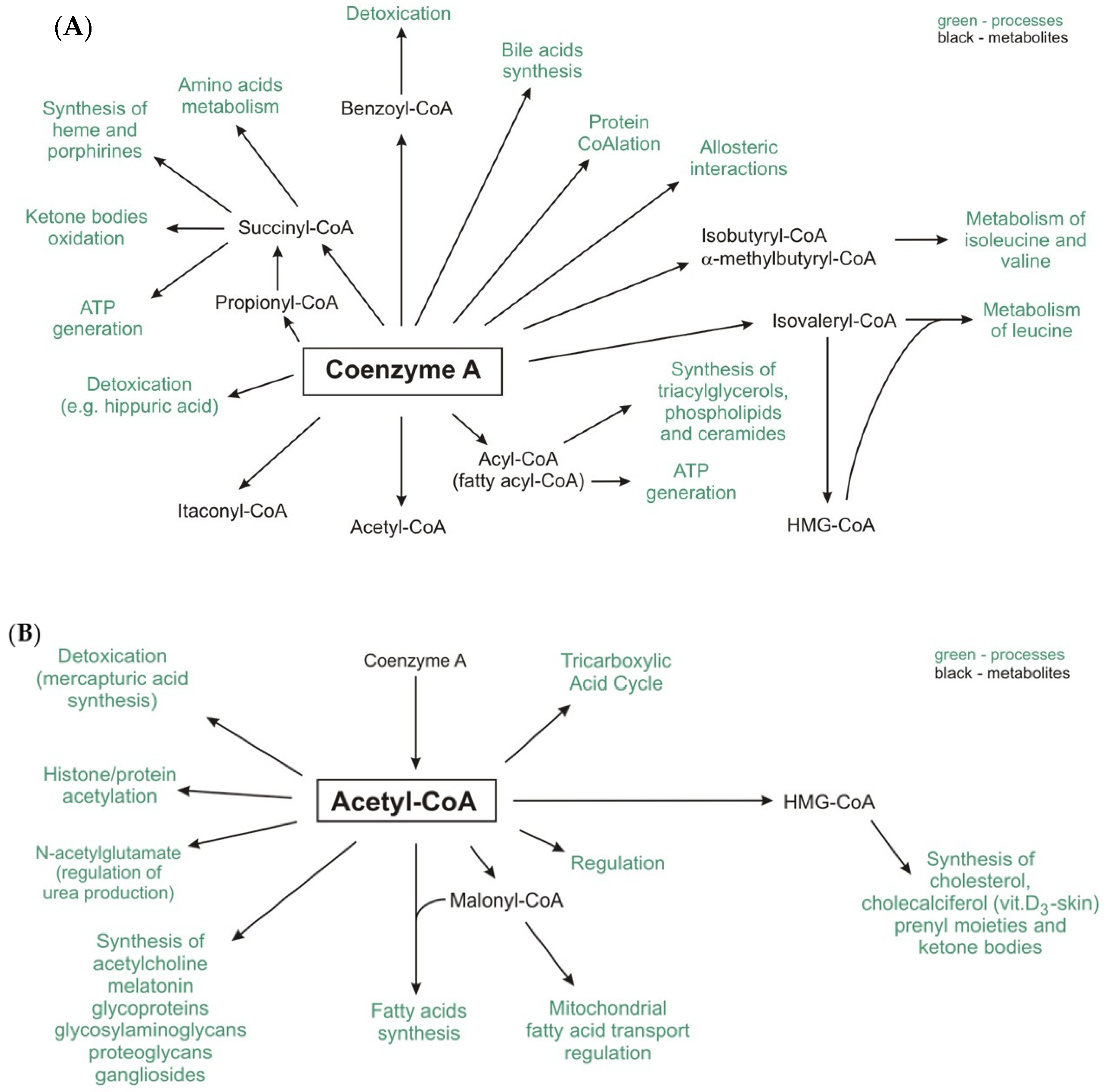
Itaconyl-CoA, a derivative of a newly discovered mammalian metabolite, itaconate, inhibits B12-dependent methylmalonyl-CoA mutase [14].
Among the abovementioned compounds, acetyl-CoA is the central and most important metabolite and forms an intersection between the anabolic and catabolic pathways [15]. Moreover, other important metabolites, such as malonyl-CoA (a substrate of lipogenesis and regulator of fatty acid oxidation) and 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) (a substrate for cholesterol and ketone bodies synthesis), are formed from acetyl-CoA (Figure 1B) [15][16].
The pool of CoA-SH in the cell is replenished by the enzymes that release it from thioester compounds, e.g., citrate synthase, acyl-coenzyme A: cholesterol acyltransferase (ACAT), many acyl- and acetyltransferases, acyl-CoA thioesterases, fatty acid synthase (FASN), fatty acid elongase (ELOVL), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), and CPT1 (Table 2), [17][18][19][20][21]. Notably, changes in the CoA-SH/acetyl-CoA ratio affect not only the regulation of energy metabolism but also the regulation of other cellular processes, such as autophagy, mitosis, and cell death [20][22].
2. CoA and Its Precursor Pantethine as Circulating Lipid-Lowering Supplemental Agents
Since CoA-SH plays a key role in lipid metabolism, especially in fatty acid oxidation, scientists have hypothesized that CoA-SH or its precursor supplementation might reduce circulating lipid concentration. Indeed, it has been reported that the combination of CoA-SH (used at pharmacological doses) with a moderate dose of a statin was more effective in curing patients with mixed dyslipidemia than statin monotherapy [23]. Moreover, it has been shown that combined CoA-SH and statin therapy was more effective in improving triacylglycerol, total cholesterol, and non-HDL-cholesterol concentrations than statin alone in patients with metabolic syndrome and mixed hyperlipidemia [23].
Much more attention has been given to pantethine (a CoA-SH precursor) as a circulating lipid-lowering compound than to CoA-SH. Pantethine, a stable form of pantetheine (two molecules of pantetheine linked by a disulfide bridge) as a dietary supplement (used at a pharmacological dose of 600–1200 mg per day), lowers elevated levels of total cholesterol, LDL-cholesterol, triacylglycerol, and non-HDL-cholesterol concentrations [24][25][26]. The effect of orally administered pantethine also results in: (a) an increase in HDL cholesterol concentration; and (b) normalization of apolipoprotein B (apo B) and apolipoprotein A (apoA); however, the effect depends on the type of dyslipidemia [27][28][29].
Some data suggest that CoA-SH (used at 400 U per day) can improve blood triacylglycerol and lipoprotein concentrations (total cholesterol and non-HDL cholesterol were significantly reduced, and HDL cholesterol was increased) to a greater extent than pantethine (used at 600 U per day) [30].
Taken together, the results published thus far indicate that CoA-SH and pantethine can be useful in lowering elevated levels of circulating lipids in some diseases. This effect is most impressive when pantethine side effect and toxicity (practically none, when used at concentrations effectively lowering blood lipid concentration) are compared with commonly used drugs that lower circulating lipids (for instance, statins). However, the effect of CoA-SH or pantethine on circulating lipids is relatively slow. Usually, a maximal effect is observed at 4 months but may take up as long as 6–9 months.
The exact molecular mechanism of action of CoA-SH and pantethine on blood lipid concentration is unknown. Although pantethine is a precursor for vitamin B5 synthesis and intake of the pharmacological dose of pantethine results in higher circulating vitamin B5 concentration, the production of vitamin B5 is not the mechanism of action because intake of panthotenic acid does not have the same effect on serum lipid concentration [2]. Pantethine-induced inhibition of acetyl-CoA carboxylase by cysteamine, the product of pantethine and CoA degradation, and inhibition of HMG-CoA reductase and cholesterol synthesis in isolated hepatocytes by pantethine may explain, at least in part, the fact that pantethine (and possibly CoA-SH) administration in pharmacological doses is effective in reducing plasma triacylglycerol and cholesterol (total cholesterol, LDL-cholesterol, and non-HDL-cholesterol) concentrations [31][32][33].
Pantethine (specifically, the cysteamine formed from pantethine) inhibition of acetyl-CoA carboxylase decreases the level of malonyl-CoA, which is (a) a substrate for fatty acid synthesis; and (b) an allosteric inhibitor of CPT1, a key regulator of fatty acid oxidation. Consequently, this reduction of malonyl-CoA leads to (a) a decrease in fatty acid synthesis; and (b) an increase in fatty acid oxidation in mitochondria. In turn, plasma lipids are affected, especially by triacylglycerol-lowering effects.
However, another mechanism is not excluded. Considering that (a) gut microbiota (especially bacterial strains such Lactobacillus and Bifidobacterium) aid in decreasing lipids in hyperlipidemic patients; and (b) pantethine promotes the survival and growth of various beneficial gut bacteria; it has been suggested that microbiota can contribute (at least in part) to a possible mechanism of pantethine action on circulating lipids [26][32][33][34][35][36][37].
Pantethine supplementation also has some beneficial effects on parameters associated with platelet lipid composition and cell membrane fluidity. In diabetic patients, the lipid composition of platelets is significantly different than that of healthy subjects. Supplementation with pantethine normalizes platelet fatty acid composition to a control value, leading to a significant reduction in platelet hyperaggregation [38]. Moreover, pantethine inhibits lipid peroxidation of the LDL-cholesterol fraction and consequently reduces lipid deposition, intimal thickening, and fatty streak formation in the aorta and coronary artery [39]. The metabolic effects of pantethine are summarized in Figure 2.
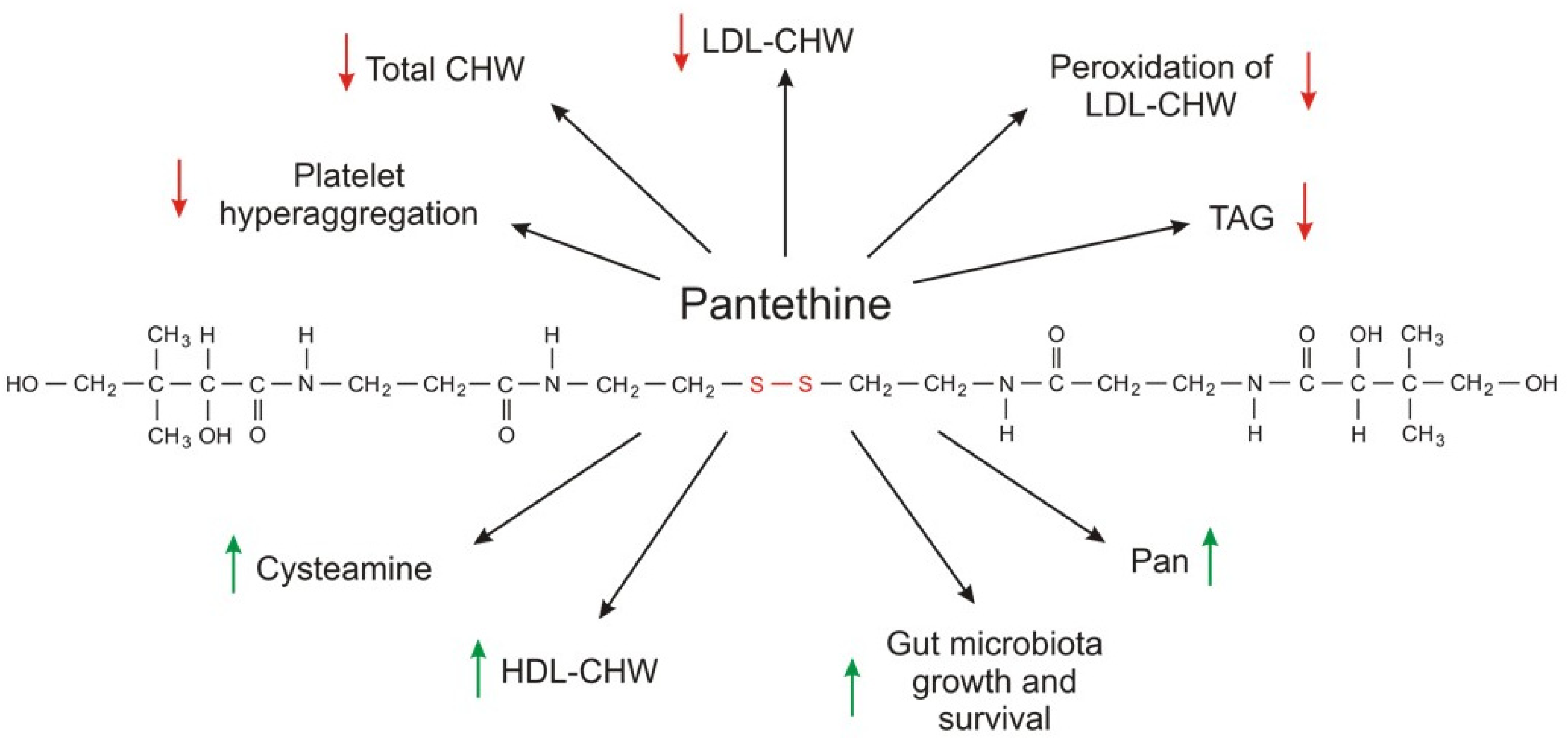
Although several clinical trials have shown that CoA-SH and especially pantethine used at pharmacological doses reduce circulating lipid levels in patients with dyslipidemia associated with different pathologies, it seems that additional studies are necessary to determine whether CoA-SH or pantethine supplementation has a beneficial effect on cardiovascular risk markers independently of or in combination with a healthy diet. Moreover, further research is also needed to explain the exact molecular mechanism of CoA-SH and pantethine action on circulating lipid concentration. At present, it is safe to say that administration of CoA-SH is not entirely different from the administration of pantethine because in the end both compounds are converted to Pan and cysteamine.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21239057
References
- Lykstad, J.; Sharma, S. Biochemistry, Water Soluble Vitamins; StatPearls Publishing: Treasure Island, FL, USA, 2019.
- Miller, J.W.; Rucker, R.B. Pantothenic Acid. Present Knowledge in Nutrition, 10th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 375–390.
- Gheita, A.A.; Gheita, T.A.; Kenawy, S.A. The potential role of B5: A stitch in time and switch in cytokine. Phyther. Res. 2020, 34, 306–314.
- Patassini, S.; Begley, P.; Xu, J.; Church, S.J.; Kureishy, N.; Reid, S.J.; Waldvogel, H.J.; Faull, R.L.M.; Snell, R.G.; Unwin, R.D.; et al. Cerebral Vitamin B5 (D-Pantothenic Acid) Deficiency as a Potential Cause of Metabolic Perturbation and Neurodegeneration in Huntington’s Disease. Metabolites 2019, 9, 113.
- Yale, K.; Juhasz, M.; Mesinkovska, N.A. Medication-Induced Repigmentation of Gray Hair: A Systematic Review. Ski. Appendage Disord. 2020, 6, 1–10.
- Ismail, N.; Kureishy, N.; Church, S.J.; Scholefield, M.; Unwin, R.D.; Xu, J.; Patassini, S.; Cooper, G.J.S. Vitamin B5 (D-pantothenic acid) localizes in myelinated structures of the rat brain: Potential role for cerebral vitamin B5 stores in local myelin homeostasis. Biochem. Biophys. Res. Commun. 2020, 522, 220–225.
- Ridgway, N.D.; Mcleod, R.S. (Eds.) Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier: Amsterdam, The Netherlands, 2016.
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109.
- Theodoulou, F.L.; Sibon, O.C.M.; Jackowski, S.; Gout, I. Coenzyme A and its derivatives: Renaissance of a textbook classic. Biochem. Soc. Trans. 2014, 42, 1025–1032.
- Van der Sluis, R.; Erasmus, E. Xenobiotic/medium chain fatty acid: CoA ligase—A critical review on its role in fatty acid metabolism and the detoxification of benzoic acid and aspirin. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1169–1179.
- Gout, I.; Coenzyme, A. A protective thiol in bacterial antioxidant defence. Biochem. Soc. Trans. 2019, 47, 469–476.
- UniProt. Available online: https://www.uniprot.org/ (accessed on 25 September 2020).
- Bhandary, S.; Aguan, K. Pyruvate dehydrogenase complex deficiency and its relationship with epilepsy frequency—An overview. Epilepsy Res. 2015, 116, 40–52.
- Ruetz, M.; Campanello, G.C.; Purchal, M.; Shen, H.; McDevitt, L.; Gouda, H.; Wakabayashi, S.; Zhu, J.; Rubin, E.J.; Warncke, K.; et al. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science 2019, 366, 589–593.
- Pietrocola, F.; Galluzzi, L.; Pedro, J.M.B.-S.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821.
- Foster, D.W. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J. Clin. Investig. 2012, 122, 1958–1959.
- Zabielska, J.; Sledzinski, T.; Stelmanska, E. Acyl-coenzyme A: Cholesterol acyltransferase inhibition in cancer treatment. Anticancer Res. 2019, 39, 3385–3394.
- Stelmanska, E.; Swierczynski, J. Up-regulation of lipogenic enzyme genes expression in inguinal white adipose tissue of female rats by progesterone. J. Steroid Biochem. Mol. Biol. 2013, 134, 37–44.
- Swierczynski, J.; Hebanowska, A.; Sledzinski, T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J. Gastroenterol. 2014, 20, 2279–2303.
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131.
- Bekeova, C.; Anderson-Pullinger, L.; Boye, K.; Boos, F.; Sharpadskaya, Y.; Herrmann, J.M.; Seifert, E.L. Multiple mitochondrial thioesterases have distinct tissue and substrate specificity and CoA regulation, suggesting unique functional roles. J. Biol. Chem. 2019, 294, 19034–19047.
- Mariño, G.; Pietrocola, F.; Eisenberg, T.; Kong, Y.; Malik, S.A.; Andryushkova, A.; Schroeder, S.; Pendl, T.; Harger, A.; Niso-Santano, M.; et al. Regulation of Autophagy by Cytosolic Acetyl-Coenzyme A. Mol. Cell 2014, 53, 710–725.
- Lai, J.; Wu, B.; Xuan, T.; Liu, Z.; Chen, J. Efficacy and tolerability of adding coenzyme A 400 U/d capsule to stable statin therapy for the treatment of patients with mixed dyslipidemia: An 8-week, multicenter, double-Blind, randomized, placebo-controlled study. Lipids Health Dis. 2014, 13.
- Donati, C.; Barbi, G.; Cairo, G.; Prati, G.F.; Degli Esposti, E. Pantethine improves the lipid abnormalities of chronic hemodialysis patients: Results of a multicenter clinical trial. Clin. Nephrol. 1986, 25, 70–74.
- Rumberger, J.A.; Napolitano, J.; Azumano, I.; Kamiya, T.; Evans, M. Pantethine, a derivative of vitamin B5 used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low– to moderate–cardiovascular risk North American subjects: A triple-blinded placebo and diet-controlled investig. Nutr. Res. 2011, 31, 608–615.
- Evans, M.; Rumberger, J.; Azumano, I.; Napolitano, J.; Citrolo, D.; Kamiya, T. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: A triple-blinded placebo and diet-controlled investigation. Vasc. Health Risk Manag. 2014, 10, 89.
- Gaddi, A.; Descovich, G.C.; Noseda, G.; Fragiacomo, C.; Colombo, L.; Craveri, A.; Montanari, G.; Sirtori, C.R. Controlled evaluation of pantethine, a natural hypolipidemic compound, in patients with different forms of hyperlipoproteinemia. Atherosclerosis 1984, 50, 73–83.
- Bertolini, S.; Donati, C.; Elicio, N.; Daga, A.; Cuzzolaro, S.; Marcenaro, A.; Saturnino, M.; Balestreri, R. Lipoprotein changes induced by pantethine in hyperlipoproteinemic patients: Adults and children. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 630–637.
- Murai, A.; Miyahara, T.; Tanaka, T.; Sako, Y.; Nishimura, N.; Kameyama, M. The effects of pantethine on lipid and lipoprotein abnormalities in survivors of cerebral infarction. Artery 1985, 12, 234–243.
- Chen, Y.Q.; Zhao, S.P.; Zhao, Y.H. Efficacy and tolerability of coenzyme A vs. pantethine for the treatment of patients with hyperlipidemia: A randomized, double-blind, multicenter study. J. Clin. Lipidol. 2015, 9, 692–697.
- Cighetti, G.; Del Puppo, M.; Paroni, R.; Galli, G.; Kienle, M.G. Effects of pantethine on cholesterol synthesis from mevalonate in isolated rat hepatocytes. Atherosclerosis 1986, 60, 67–77.
- Cighetti, G.; Del Puppo, M.; Paroni, R.; Kienle, M.G. Modulation of HMG-CoA reductase activity by pantetheine/pantethine. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1988, 963, 389–393.
- McCarty, M.F. Inhibition of acetyl-CoA carboxylase by cystamine may mediate the hypotriglyceridemic activity of pantethine. Med. Hypotheses 2001, 56, 314–317.
- Jones, M.L.; Tomaro-Duchesneau, C.; Martoni, C.J.; Prakash, S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin. Biol. Ther. 2013, 13, 631–642.
- Craig, J.A.; Snelll, E.E. The comparative activities of pantethine, pantothenic acid, and coenzyme A for various microorganisms. J. Bacteriol. 1951, 61, 283–291.
- Gyorgy, P.; Rose, C.S. Further observations on the metabolic requirements of Lactobacillus bifidus var. pennsylvanicus. J. Bacteriol. 1955, 69, 483–490.
- Sultanbawa, Y. Effects of bifidogenic factors on growth of bifidobacterium bifidum in cultured milk yoghurt. J. Natl. Sci. Found. Sri Lanka 2006, 34, 205.
- Hiramatsu, K.; Nozaki, H.; Arimori, S. Influence of pantethine on platelet volume, microviscosity, lipid composition and functions in diabetes mellitus with hyperlipidemia. Tokai J. Exp. Clin. Med. 1981, 6, 49–57.
- Bittolo Bon, G.; Cazzolato, G.; Zago, S.; Avogaro, P. Effects of pantethine on in-vitro peroxidation of low density lipoproteins. Atherosclerosis 1985, 57, 99–106.
