Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Hormones produced by adipocytes, leptin and adiponectin, are associated with the process of carcinogenesis. Both of these adipokines have well-proven oncologic potential and can affect many aspects of tumorigenesis, from initiation and primary tumor growth to metastatic progression. Involvement in the formation of cancer includes interactions with the tumor microenvironment and its components, such as tumor-associated macrophages, cancer-associated fibroblasts, extracellular matrix and matrix metalloproteinases.
- leptin
- adiponectin
- tumor microenvironment
- epithelial–mesenchymal transition
1. Tumor Microenvironment
TME is a complex of a variety of cells and molecules surrounding tumors. This system includes tumor endothelial cells, tumor stromal cells with cancer-associated fibroblasts (CAFs) and cancer-associated adipocytes (CAAs), normal epithelial cells, immune cells with tumor-associated macrophages (TAMs), signaling molecules, blood vessels and a non-cellular part called the extracellular matrix (ECM). Closely related components and tumors constantly interact and affect each other, taking part in the growth of cancer cells and tumor progression [57,58]. Tumor factors can regulate the expression of adipokines with oncologic potential, such as leptin and adiponectin, which interact with cancer cells through TME as well [59,60]. Figure 3 illustrates the composition of TME.
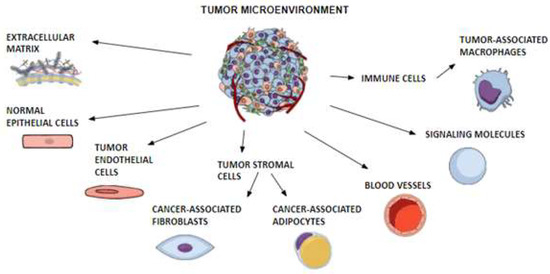
Figure 3. Composition of the tumor microenvironment. A schematic diagram shows the different components of the tumor microenvironment. The dynamic and bidirectional interactions of tumor cells with their microenvironment, consisting of cellular and non-cellular parts, are fundamental to the stimulation of tumor growth, invasion and metastasis. The figure was created by mindthegraph.com (accessed on 6 March 2023).
1.1. TME
1.1.1. Leptin and TME
Leptin is associated with the cellular and molecular parts of TME and can be affected through direct and indirect mechanisms that could lead to tumor cell invasion and distant metastasis [4,61,62]. Leptin treatment directly affects pro-inflammatory, angiogenic and fibrotic factors in TME [63]. The concentration of leptin is higher in plasma samples from TME blood than in plasma from peripheral blood samples of obese patients with estrogen receptor-positive breast cancer. With overexpression of the leptin gene in breast cancer tissue [64,65].
1.1.2. Adiponectin and TME
Adiponectin is the most abundant adipokine in TME. The role of adiponectin in TME is not yet fully understood. There is an inverse correlation between circulating adiponectin and the various tumor antioxidant markers [66]. CAAs are a noted cause of decreased adiponectin secretion in humans [65,67]. In colorectal cancer, adiponectin modulates the inflammatory responses and influences the TME, which eventually defines the destiny of tumors [68]. This adipokine, together with the n-6 or n-3 polyunsaturated fatty acids (PUFAs) produced by periprostatic adipose tissue in prostatic cancer, has anti-tumoral effects [69]. Lower expression of adiponectin, AdipoR2, leptin and ObRs in the breast TME might be indicators of more aggressive breast cancer phenotypes [65,70,71]. The interactions of adipokines with TME are summarized in Table 1.
Table 1. Leptin and adiponectin interactions with tumor microenvironment.
| Component | Adipokine | Cancer Types | Effect | Mechanisms | References |
|---|---|---|---|---|---|
| TME | ↑ Leptin | [64] | |||
| ↓ Adiponectin | [69] | ||||
| CAFs | Leptin | Breast cancer | ↑ Proliferation ↑ Migration ↑ Invasion |
[9,72] | |
| NSCLC | ↑ Malignancy | [73] | |||
| Pancreatic cancer | ↑ Invasion | [74] | |||
| Adiponectin | Colon cancer | ↑ Angiogenesis ↑ Tumor growth ↑ Proliferation ↑ Migration ↑ Invasion |
[75] [76] |
||
| TAMs | Leptin | Breast cancer | ↑ Malignancy ↑ Tumor growth ↑ Progression |
[77,78,79] | |
| Melanoma | ↑ Metastasis | [80] | |||
| Gallbladder cancer | ↑ Invasion ↑ Migration |
[81] | |||
| Colorectal cancer | ↑ Tumor growth | [82] | |||
| Adiponectin | Melanoma | ↓ Tumor growth | [83] | ||
| Lung cancer | ↓ Tumor growth | [83] | |||
| Rhabdomyosarcoma | ↓ Tumor growth | [84] | |||
| MMPs | Leptin | Gastric cancer | ↑ Invasion ↑ Metastasis |
MMP-1 | [85] |
| Breast cancer | ↑ Progression | MMP-2 MMP-9 |
[64,86,87] | ||
| Oesophageal cancer | ↑ Invasion | MMP-2 MMP-9 |
[88] | ||
| Gallbladder cancer | ↑ Metastasis | MMP-3 MMP-9 |
[89] | ||
| Ovarian cancer | ↑ Migration ↑ Invasion |
MMP-7 | [90] | ||
| Colon cancer | ↑ Progression | MMP-7 | [91] | ||
| Pancreatic cancer | ↑ Migration ↑ Invasion |
MMP-13 | [92] | ||
| Adiponectin | NSCLC | ↑ Invasion | MMP-1 MMP-2 MMP-9 MMP-14 |
[93] | |
| RCC | ↓ Tumor growth ↓ Metastasis ↓ Angiogenesis |
MMP-2 MMP-9 |
[94] | ||
| Liver cancer | ↓ Tumor growth ↓ Metastasis |
MMP-9 | [95] | ||
| Oesophageal cancer | ↓ Invasion | MMP-2 MMP-9 |
[88] | ||
Abbreviations: CAFs, cancer-associated fibroblasts; MMPs, matrix metalloproteinases; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; TAMs, tumor-associated macrophages; TME, tumor microenvironment.
1.2. CAFs
CAFs play a pro-tumorigenic role by secreting various growth factors, cytokines and chemokines, as well as by degrading the ECM [96]. Adipocytes may be one of the origins of CAFs. The adipocytic phenotype may convert to CAFs by co-culturing with cancer cells and promoting their malignancy [97].
1.2.1. Leptin and CAFs
In breast malignancy, leptin is secreted by CAFs, and it is responsible for the bidirectional interactions between CAFs and breast cancer cells, leading to the proliferation, migration and invasiveness of breast cancer cells [9,65,72]. Leptin produced by CAFs also leads to increased malignancy in non-small-cell lung cancer (NSCLC) cells via MAPK/ERK1/2 and PI3K/AKT signaling pathways in a paracrine manner [73]. Moreover, CAFs are the most abundant cells in the stroma of pancreatic tumors, so it is possible that, in the same way CAFs-secreted leptin could be involved in the invasion of pancreatic cancer cells [74].
1.2.2. Adiponectin and CAFs
Adiponectin enhances tumor angiogenesis and tumor growth by inducing stromal fibroblast senescence through activation of p53 and p16-dependent pathways and by stimulating CXC chemokine ligand 1 (CXCL1) secretion from cancer cells, a key regulator of granulocyte recruitment. Thus, adiponectin deficiency could result in inhibition of tumor progression through reduction in stromal fibroblast senescence, in subcutaneous and metastasis tumor tissue, and discontinuing angiogenesis [75].
Carnitine palmitoyl transferase IA (CPT1A) is a rate-limiting enzyme of fatty acid oxidation (FAO), whose upregulation in CAFs promotes the proliferation, migration and invasion of colon cancer cells by increasing the ability of CAFs to release cytokines such as chemokine (C-C motif) ligand 2 (CCL2), vascular endothelial growth factor A (VEGF-A) and matrix metalloproteinase-2 (MMP-2). CAFs can induce lower CPT1A expression by reducing the secretion of adiponectin [76].
1.3. TAMs
TAMs represent one of the major types of immune cells infiltrating tumors. Due to their role and polarization states, two types of macrophages are distinguished: classically activated M1 macrophages and alternatively activated M2 macrophages. The M1 macrophages are implicated in the inflammatory response, elimination of pathogens and anti-tumor functions. The M2 macrophages, on the other hand, influence the anti-inflammatory response and, with their pro-tumorigenic properties, can promote the occurrence and invasion of tumor cells, leading to tumor progression [98].
Ongoing studies have shown that the TAM population is in a state of constant transition between the M1 and M2 types. The differentiation and polarization of TAMs and the proportion of each form are determined by multiple TME cytokines, chemokines, growth factors and other signals. Moreover, adipose tissue also recruits macrophages, whereas the tumor also recruits adipose stroma cells, showing a strong relationship between them. Though TAMs are able to exhibit either polarization phenotype, they closely resemble M2 macrophages as crucial modulators of TME. TAMs influence tumor progression, including cancer initiation and promotion, tumor angiogenesis, immune regulation and metastasis [99,100,101]. TAMs can also demonstrate antitumor activity. Hence, in response to microenvironmental signals, TAMs can have a dual effect on tumor growth and progression [102]. Moreover, TAMs are involved in tumor responses to therapy and modulate the efficacy of anticancer therapies such as chemotherapy, tumor irradiation, vascular-targeted therapies, targeted therapies by monoclonal antibodies and immunotherapies [103]. Nevertheless, TAMs are also considered therapeutic targets, with different types of molecular agents against TAMs as potential anti-cancer approaches [100,104].
4.3.1. Leptin and TAMs
Leptin, through its connection to ObRs, which are present on the surface of inflammatory cells, regulates macrophage polarization and elevates the expression of different cytokines in TAMs. Knockdown of ObRs impacts the macrophage phenotype in TME, inhibiting breast cancer malignancy [77]. Leptin activates M2 macrophages and enhances the production of the cytokines IL-6, IL-8, IL-12, IL-18 and TNF-α, while inhibiting IL-10 and IL-4 [39,63]. Upregulated IL-18 expression, both in TAMs via activation of NF-κB/NF-κB1 and in breast cancer cells by activation of PI3K-AKT/activating transcription factor 2 (ATF-2) signaling pathways induced by leptin and IL-8 production in M2 macrophages stimulated by leptin, significantly promotes the migration and invasion of breast cancer cells. Apart from breast cancer, it is proven that IL-18 participates in the pathogenesis and metastasis of gastric cancer and melanoma [78,79].
Leptin might mediate the link between CAAs and M2 macrophages in metastasis [80]. Via STAT3, it promotes the polarization of M2 macrophages and enhances gallbladder cancer cell invasion and migration [81]. In colorectal cancer macrophage-specific metabolites, itaconate can exert cancer-promoting effects in M2 macrophages through downregulation of peroxisome proliferator-activated receptor gamma (PPARϒ), a cellular pathway that is regulated by leptin and acts as a tumor-suppressing factor. Also in colorectal cancer, leptin affects macrophage polarization [82].
1.3.2. Adiponectin and TAMs
Adiponectin is a significant regulator of macrophage proliferation, polarization and function in inflammation. The strengthened tumor growth seen in adiponectin deficiency is likely due to the reduction in macrophage recruitment to the tumor rather than enhanced angiogenesis [83]. Undeniable adiponectin promotes the polarization of M2 macrophages [105,106,107]. It acts as an anti-inflammatory factor by suppressing M1 macrophage activation and downregulating proinflammatory cytokines via NF-kB activation, together with promoting M2 macrophage proliferation and expression of anti-inflammatory M2 macrophage markers via AMPK and AKT/PI3K-dependent mechanisms [108]. Furthermore, adiponectin regulates JmJC family histone demethylase 3 (JMJD3), which is necessary for M2 polarization, as another anti-inflammatory mechanism [109]. Adiponectin deficiency plays an important role in restraint tumor growth by reprogramming TAMs into M1 macrophages via suppressing p38 MAPK phosphorylation and partially mediating adiponectin-induced TAM polarization, which consequently limits tumor growth [14,84]. In the other direction, M1 macrophages also affect the action of adiponectin by reducing the expression of AdipoRs [37].
2. Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are a group of proteolytic enzymes that degrade ECM and are implicated in migration, invasion and metastasis; thereby, MMPs play important roles in cancer progression. Tissue inhibitors of matrix metalloproteinases (TIMPs) affect tumor cell invasiveness and the formation of distant metastases [110]. Therefore, MMPs have the potential to be diagnostic and prognostic markers along with therapeutic targets in cancer patients [111]. MMPs and TIMPs take part in adipogenesis [112,113,114] and adipocytes exert an important role in modifying the ECM through the secretion of MMPs such as MMP-1, MMP-7, MMP-10, MMP-11 and MMP-14 [61]. In obesity, adipogenesis undergoes dynamic remodeling, which is related to the turnover of ECM components [115]. In obese states, serum concentrations of MMP-2 and MMP-9 are elevated [116].
2.1. Leptin and MMPs
Many studies have reported that one of the leptin-induced cancer-cell invasion mechanisms is upregulating MMP expression. Leptin is involved in hepatocellular carcinoma development through its interaction with MMPs in the carcinogenic microenvironment [117]. This hormone promotes gastric cancer cell invasion by upregulating membrane type 1-matrix metalloproteinase (MT1-MMP) expression, and its overexpression positively correlates with clinical stage and lymph node metastasis in gastric cancer [85]. Leptin induces breast cancer progression by activating the expression of MMP-2 and MMP-9 [64,86,87]. Also, in oesophageal cancer, it stimulates the release of MMP-2 and MMP-9, increasing the invasiveness of cancer cells [88]. This adipokine may also be involved with the metastasis of gallbladder cancer as a result of increasing levels of MMP-3 and MMP-9 [89].
MMP-7 is considered to play an important role in the activation of various MMPs. High blood levels of MMP-7 are associated with the tumor progression of colorectal cancer and positively correlate with the advanced stage of ovarian cancer. Leptin increases MMP-7 expression and subsequent migration and invasion of ovarian cancer cell lines via ObRb, ERK1/2 and JNK1/2 activation signaling pathways and ObRb gene silencing suppresses leptin-induced MMP-7 expression [118]. In colon cancer, leptin-mediated expression of MMP-7 and cell invasion follow MAPK/ERK and PI3K/AKT signaling pathways. Moreover, leptin-induced MMP-7 expression activates MMP-2 and MMP-9. All of this proves leptin’s modulatory effects in the regulation of colon cancer progression [90,91].
It is also shown that leptin promotes cell invasion and migration through an increase in MMP-13 production, which serves as a downstream effector of the leptin-JAK2/STAT3 cascade responsible for cell invasion in pancreatic cancer cells. The tumoral expression of ObRb and MMP-13 correlates with lymph node metastasis [92].
Leptin may also contribute to the migration and invasion abilities of non-cancer cells, for example, endometriotic cells via the up-regulation of MMP-2 through an ObR-dependent JAK2/STAT3 signaling pathway [119] and human trophoblastic cells through MMP-14 overexpression, requiring the crosstalk between neurogenic locus notch homolog protein 1 (Notch1) and PI3K/AKT signaling pathways [120] as well as by regulating the expressions of MMP-9, TIMP1, TIMP2 and E-cadherin [121].
2.2. Adiponectin and MMPs
The influence of adiponectin on the expression and activity of MMPs and TIMPs is less well researched, but it plays an important role in ECM modulation [122,123,124]. Adiponectin enhances the production of MMPs such as MMP-1, MMP-2, MMP-3, MMP-9 and MMP-13 [37,125,126,127,128] and reduces TIMP1 activity—an inhibitor of MMP-9 [42,129]. Also, MMPs provide feedback on adiponectin, for example, MMP-12 induces globular adiponectin production from full-length adiponectin [130].
Adiponectin is significantly more expressed in metastatic NSCLC than in NSCLC without metastasis. In the A549 cell culture NSCLC model, transfection with adiponectin successfully increased the expression levels of MMP-1, MMP-2, MMP-9 and MMP-14, demonstrating an adiponectin-MMPs-involved mechanism in NSCLC invasion [93]. Adiponectin, via AdipoR1, inhibits mTOR through AMPK activation in renal cell carcinoma (RCC), suppresses vascular endothelial growth factor (VEGF), MMP-2 and MMP-9 and increases TIMP-1 and TIMP-2 secretion, resulting in decreased growth, dissemination and angiogenesis of RCC [94]. In liver cancer, this adipocyte-derived hormone impacts cancer growth and metastasis by downregulating gene expression levels of Rho-associated protein kinase (ROCK), IFN-inducible protein 10 (IP10), angiopoietin 1 and MMP-9 in liver tumors, as well as downregulating ROCK/IP10/angiopoietin 1/MMP-9/VEGF cell signaling in tumor tissue [95].
Furthermore, adiponectin can affect the interaction of leptin with MMPs. Via AMPK activation and through inhibition of JAK2/STAT3 (by promoting binding of SOCS-3 to ObRs and stimulating protein tyrosine phosphatase 1B (PTP1B) expression and activity—both negative regulators of this signal transduction pathway), adiponectin may block leptin-stimulated secretion of TIMP-1 and significantly stimulate MMP-1 activity [131,132]. By activating both a non-specific tyrosine phosphatase inhibitor and a specific PTP1B inhibitor, it significantly reduces the secretion of MMP-2 and MMP-9 from leptin-stimulated oesophageal cancer cells, inhibiting cancer invasion through this mechanism [88].
3. Epithelial–Mesenchymal Transition
Epithelial cells are organized into multicellular layers connected with each other through strong epithelial-cell junctions on both lateral sides, such as adherents junctions, tight junctions, gap junctions, and desmosomes. Another structural feature is apical–basal polarity and interaction with the underlying basement membrane. Mesenchymal cells present front-back polarity with no functional cell–cell junction components, including E-cadherin and β-catenin [133].
EMT is a cellular process whereby epithelial cells lose their characteristic polarity and cell adhesions and acquire the morphological and functional features of mesenchymal cells, which results in enhanced migratory and proliferation, apoptosis resistance, and their ability to produce ECM components. Particularly, the E-Cadherin and N-Cadherin switch and loss of E-cadherin and vimentin expression are two of the most well-defined features of EMT that can be triggered and regulated at different levels by multiple factors, including signals from TME [134,135,136]. Thus, EMT is associated with alterations of the intracellular cytoskeleton and ECM degradation, which cause local invasion and subsequent dissemination to distant tissues [137].
EMT types are specified. Type I and II EMT are associated with many physical processes: embryonic and organ development, wound healing, tissue regeneration, and fibrosis [138,139] while type III EMT is crucial for tumor malignancy and plays important roles in cancer progression [140,141]. EMT is frequently activated during metastasis and is directly linked to the acquisition of cancer stem cell (CSC) properties [142].
The most common and, at the same time, most lethal human malignancies are derived from epithelial tissues. Cancer-associated deaths are mostly caused by metastatic disease. EMT—an important phenomenon for cancer cells—is activated during either tumorigenesis or metastasis [136,143]. Further, due to the involvement of EMT in the metastatic process and the various states produced during EMT, targeting and manipulating this process provides a number of opportunities to influence cancer progression and can be used for therapeutic strategies in cancer during different procedures [143,144]. Currently, there are few clinical trials testing the therapeutic efficacy of agents specifically designed to suppress EMT program expression [141]. Regulators involved in EMT may be used as biomarkers and for therapeutic targeting [136].
3.1. Leptin and EMT
Leptin signaling activates multiple pathways and affects transcriptional factors that drive reprogramming of gene expression underlying epithelial loss and expression of mesenchymal features associated with loss of cell–cell junctions and apical-basal polarity [143,145]. Several studies describe that leptin promotes the expression of mesenchymal markers and decreases epithelial markers, in addition to promoting EMT-related processes such as cell migration and invasion and a poor prognosis in patients with numerous types of cancer [12,60,62,146].
The association between leptin and EMT has been most well studied in breast cancer. Chronic leptin treatment induces EMT in non-tumoral breast epithelial MCF10A cells, which leads to the belief that high leptin expression in normal breast tissue with the assistance of EMT contributes to a higher risk of breast cancer [147]. Indeed, leptin, through cytosolic tyrosine kinases such as steroid receptor coactivator (Src) and focal adhesion kinase (FAK) activation, promotes the expression of EMT-related transcription factors and invasion in MCF10A cells [87]. Leptin is involved in the regulation of EMT in triple-negative breast cancer (TNBC), and EMT regulators are major targets of TNBC [148,149]. In breast cancer cells (BCCs), leptin induces EMT by β-Catenin activation through AKT/glycogen synthase kinase 3 beta (GSK3β) and metastasis-associated protein 1 (MTA1)/Wnt family member 1 (Wnt1) pathways, as well as functional interactions between leptin, Wnt1 signaling components and MTA1—an important modifier of Wnt1 signaling [150]. Other research demonstrates that leptin-induced EMT in BCCs requires IL-8 activation via the PI3K/AKT signal pathway [151]. Further studies also suggest that leptin promotes EMT in BCCs via the activation of the PI3K/AKT signaling pathway but also via the overexpression and activation of pyruvate kinase M2 (PKM2) [152].
Another leptin signaling pathway for EMT is the transforming growth factor beta 1 (TGFB1) pathway, a central player in EMT that interacts with other EMT signaling pathways. Support for breast cancer invasiveness and CSC behavior by leptin is mediated through the binding of TGFB1 to its receptor. Further, antagonizing the TGFB-TGFB-receptor interaction degrades the EMT-promoting effects of leptin [142]. BCCs co-cultured with adipose stromal/stem cells isolated from obese women (obASCs) demonstrated enhanced expression of EMT and metastasis genes (SERPINE1, MMP-2, IL-6), and knockdown of leptin produced by obASCs significantly reduced tumor volume and decreased the number of metastatic lesions to the lung and liver [86].
The stromal cell-derived factor 1 (SDF-1) is a chemokine frequently produced in large amounts by target organs where metastasis occurs. Chemokine receptor type 4 (CXCR4) is the sole receptor for SDF-1, which was also recently described as a marker of EMT. Leptin induces tumor dissemination and metastasis of BCCs to bone tissue by activating the SDF-1/CXCR4 axis, and upregulation of CXCR4 contributes to bone metastasis and poor survival. Moreover, leptin downregulates expression of the epithelial marker E-cadherin and upregulates expression of the mesenchymal marker vimentin in BCCs, and inhibition of ObRs in BCCs significantly reduces the incidence of leptin-induced EMT [153].
In esophageal adenocarcinoma (EAC), leptin produced by peritumoral adipose tissue with increased cell diameter upregulates expression of EMT markers such as alpha-smooth muscle actin (α-SMA) and E-cadherin and thus may promote extension and penetration by cancer cells into neighboring tissues [154]. Snail is a zinc-finger transcriptional repressor that induces EMT and downregulates E-cadherin expression. It is a metastatic suppressor that is lost and shifts to N-cadherin, which is one of the typical features of EMT [132]. In gastric cancer, leptin increases the mRNA and protein levels of those EMT markers—Snail and N-cadherin—inducing EMT in such a manner [155].
In cholangiocarcinoma, leptin significantly stimulates EMT by provoking cell migration and invasion, impacting multiple levels of EMT promoters (reducing E-cadherin and β-catenin expression in addition to enhancing vimentin and N-cadherin expression) along with the proangiogenic capability of cholangiocarcinoma cells through the microRNA-122/PKM2 axis [156]. Leptin can regulate EMT through the activation of the Hedgehog (Hh) pathway, which induces hepatic stellate cells acquisition/maintenance of a myofibroblastic phenotype [157]. Leptin significantly increases tumor necrosis factor alpha (TNF-α) secretion through the activation of p38 and JNK/MAPK [28] and TNF-α can induce cancer invasion and metastasis associated with EMT in colorectal cancer [158], suggesting a potential effect of leptin on EMT in colorectal cancer as well.
A study on A549 human lung cancer cell lines shows that leptin can significantly enhance the expression of transforming growth factor beta (TGF-β), which is a direct inducer of EMT [159]. Moreover, leptin, through a mechanism dependent on the activation of the ERK signaling pathway, increases EMT-induced tumor phenotypes in lung cancer cells too [160].
Research in prostate cancer cells demonstrates that leptin, by stimulating the STAT3 signaling pathway, promotes EMT and migration of prostate cancer cells [161]. Leptin treatment upregulates EMT in ovarian and pancreatic cancer cell lines as well [148].
MMPs can induce EMT in two ways: directly by degrading adherents and tight junction proteins (MMP-2, MMP-9) [59,87,162] and indirectly by TGF-β and TGF-β-related protein activation (MMP-2, MMP-9, MMP-13 and MMP-14) [59,138,163]. In addition, M2 macrophages could play key roles in cancer progression, including the promotion of EMT. Thus, leptin-induced stimulation of M2 macrophages and MMPs affects EMT [4].
3.2. Adiponectin and EMT
Compared to leptin, less is known about the relationship between adiponectin and EMT. Nevertheless, several researchers have attempted to investigate these relationships. Evidence suggests that adiponectin inversely correlates with cancer progression, in part due to the reversal and inhibition of EMT [60,145].
The insulin-like growth factor-I receptor (IGF-IR) contributes to the establishment and maintenance of EMT as well as the development and maintenance of CSC in breast cancer. In ERα-negative BCCs, adiponectin has an antagonistic effect on IGF-IR signaling through activation of AMPK and inhibition of mTOR signaling, indirectly blocking IGF-IR-induced EMT [164]. However, AdipoR1 can regulate EMT in breast cancer as a direct target of microRNAs (miRNAs) miR-221 and miR-222 (miR-221/222) and provides an additional node by which miR-221/222 induces BCCs EMT. In breast cancer, miR-221/222 is differentially expressed in the clinically more aggressive basal-like subtype compared to the luminal subtype, and upregulation of miR-221/222 provokes EMT, which shows that AdipoR1 may play an important role in breast cancer progression and metastasis by implication [165].
In nasopharyngeal carcinoma (NPC) patients, serum adiponectin level is inversely correlated with tumor stage, recurrence and metastasis, and low serum adiponectin level correlates with poor metastasis-free survival. EMT is involved in the invasion and migration of tumor cells, and adiponectin via AdipoR1 has a reversing impact on this process through two mechanisms. Firstly, adiponectin treatment significantly increases the expression of E-cadherin and Claudin-1 while decreasing the levels of N-cadherin, MMP-2, MMP-9, Snail, Slug and vimentin, further blocking the EMT process. Secondly, recombinant adiponectin or a specific adiponectin receptor agonist (AdipoRon) mediates the inhibitory effect on activation of NF-κB and STAT3 signaling pathways, which are leptin-induced signaling pathways intimately involved in promoting EMT and play important roles in the metastasis of NPC [166].
The results of the study on NSCLC revealed that adiponectin is an important negative regulator of NSCLC migration and invasion through the reversal of the EMT process. After adiponectin administration, NSCLC cells displayed increased epithelial marker expression and downregulation of mesenchymal marker expression. Adiponectin upregulated E-cadherin and downregulated vimentin expression. What is more, AdipoR1 or AdipoR2 knockdown eliminated the inhibitory effects of adiponectin on migration and invasion in NSCLC and EMT, which proves that both AdipoRs mediate the adiponectin-associated signaling pathways to regulate EMT [167].
In colon cancer, adiponectin reduces cell migration ability and survival rate in association with the induction of oxidative stress and the regulation of cytokine expression (IL-6, IL-8 and IL-10). Nonetheless, Western blot analysis performed on E-cadherin and vimentin, two EMT-crucial markers in carcinogenesis, indicated that adiponectin does not influence EMT transition [135,168].
Adiponectin silencing in 22RV1 cells—human prostate cancer cell—downregulates the expression of epithelial markers: E-cadherin and zonula occludens-1 (ZO-1), but upregulates the expression of mesenchymal markers: zinc finger E-box binding homeobox 1 (ZEB1), vimentin and Snail. In addition, epigenetic modifications of adiponectin are involved during the EMT process. TGFB1 treatment in 22RV cells significantly decreased the expression levels of adiponectin, suggesting that adiponectin may play an inhibitory role in EMT. It shows that silencing endogenous adiponectin could promote the proliferation and invasion of prostate cancer cells via the EMT process. In consequence, in prostate cancer, adioponectin may function as a potential tumor suppressor but is commonly downregulated by DNA promoter methylation [169].
In view of the fact that adiponectin inhibits proliferation through blocking phosphorylation of GSK-3β, preventing β-catenin activation, and nuclear translocalization in breast cancer, this effect has been investigated on GSK-3β signaling pathways in RCC cells. In RCC, adiponectin administration also inhibited the phosphorylation of GSK-3β and decreased the accumulation of β-catenin. Additionally, silencing AdipoR1 restored the expression of EMT-related proteins, so activating the adiponectin AdipoR1 axis could hinder their expression. Inhibition of GSK-3β/β-catenin pathway by adiponectin was involved in the reduction in RCC cell motility and invasiveness without an antiproliferative effect, thus downregulating the phosphorylation of GSK-3β can stop EMT [170].
As mentioned earlier, the hallmark of EMT is the upregulation of N-cadherin, followed by the downregulation of E-cadherin [134]. Although T-cadherin, as a non-classical adiponectin receptor localized on the apical cell surface, like EMT epithelial and mesenchymal E-cadherin and N-cadherin, belongs to the cadherin superfamily, due to the lack of a transmembrane and cytoskeletal domain, T-cadherin does not participate in cell-cell adhesion but plays an important role in intracellular signaling. However, studies investigating the role of T-cadherin in cancer describe T-cadherin as a tumor suppressor in many cancer types, and its loss is associated with a more aggressive course of numerous cancers, which also indicates the involvement of adiponectin in carcinogenesis and the therapeutic potential of T-cadherin [171,172].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15174250
This entry is offline, you can click here to edit this entry!
