Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Plant viruses, as obligate intracellular parasites, induce significant changes in the cellular physiology of host cells to facilitate their multiplication. These alterations often lead to the development of symptoms that interfere with normal growth and development, causing USD 60 billion worth of losses per year, worldwide, in both agricultural and horticultural crops.
- plant virus
- virus-host interaction
- cellular process
- photosynthesis
- symptoms
- mosaic
1. Introduction
Light and temperature are important environmental factors that profoundly influence plant growth, development and physiology. Interestingly, many studies have described the influence of light and temperature on development of viral symptoms. Here summarizes the effects of light and temperature conditions on the development of viral symptoms (Figure 1).
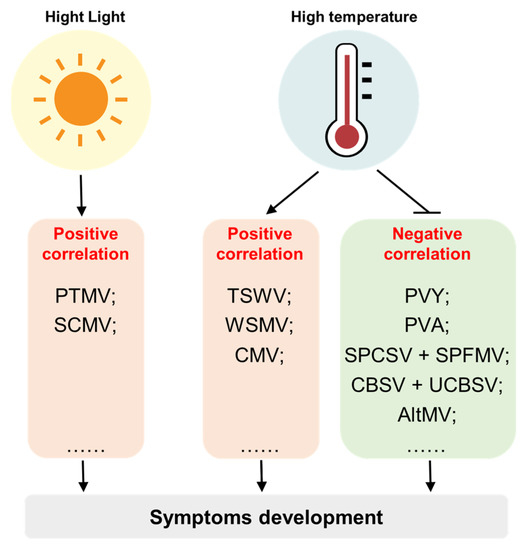
Figure 1. Environmental factors that influence the development of viral symptoms include light and temperature. In general, there is a positive correlation between light intensity and the severity of viral symptoms. However, the correlation between temperature and viral symptoms can vary depending on the specific virus and host. For some viruses like tomato spotted wilt virus (TSWV), wheat streak mosaic virus (WSMV), and cucumber mosaic virus (CMV), higher temperatures are associated with enhanced symptoms development, indicating a positive correlation. On the other hand, certain viruses like potato virus Y (PVY), potato virus A (PVA), sweet potato chlorotic stunt virus + sweet potato feathery mottle virus (SPCSV + SPFMV), cassava brown streak virus + Ugandan cassava brown streak virus (CBSV + UCBSV), and alternanthera mosaic virus (AltMV) induced symptoms show a negative correlation with temperatures, where high temperatures inhibit the symptoms development.
2. The Role of Light Conditions in Virus-Induced Symptom Development
Studies investigating the effect of light conditions on viral symptoms development have revealed intriguing findings. In the case of potato mop-top virus (PMTV) infection in tobacco plants, it was observed that the appearance of necrotic spots or small rings was delayed when there was a decrease in light intensity or photoperiod. When inoculated plants are transferred from light to darkness, necrotic rings develop, and the rate of virus accumulation increases. However, when the order of the treatments is reversed, no lesions appear. Therefore, the process of PMTV-induced lesion formation includes an early phase requiring light [1].
Similarly, a field survey conducted in an unmanaged forest revealed interesting observations regarding viral symptoms in shade-grown and sun-exposed plants. Virus-infected plant species growing under shade exhibited fewer apparent viral symptoms, while those growing in direct sunlight displayed severe chlorosis in their leaves [2].
To understand these observations, it is important to consider the role of light in photosynthesis. When a plant is infected with a virus, it can affect the photosynthetic machinery, leading to changes in energy production [3][4]. Alterations in photosynthesis can disrupt various physiological processes in plants, ultimately influencing the occurrence of symptoms. Recent research on maize plants infected with SCMV further emphasized the role of light in viral symptoms development. It was found that mosaic symptoms caused by SCMV infection only occurred under light illumination, and no mosaic symptoms were observed under dark or low-light conditions. Additionally, it was discovered that SCMV infection induced the overproduction of malate that could cause symptoms via elevating PPDK enzyme activity under light [5]. In maize plants, the key regulatory factor governing the response of PPDK to light is PPDK Regulatory Protein (PDRP) [6][7]. In the presence of light, PDRP catalyzes the dephosphorylation of PPDK, leading to the activation of its enzymatic activity, and consequently, promoting the conversion of phosphoenolpyruvate to oxaloacetate, and ultimately, to malate [6][7][8]. Conversely, under dark conditions, PDRP mediates the dephosphorylation of PPDK, resulting in a reduction of its enzymatic activity [6][7]. However, when maize plants are infected with SCMV, there is a potential that the virus might interfere with the regulatory function of PDRP. By affecting the phosphorylation state of PPDK, SCMV could disrupt the normal regulation of PPDK under light conditions, leading to abnormal levels of malate and PPDK enzyme activity. Thus, investigating whether SCMV can modulate PPDK phosphorylation and enzymatic activity via its impact on PDRP functionality represents an intriguing avenue of research. Overall, this finding provides a partial explanation for how light affects the development of viral symptoms [5].
The above research indicates the intricate relationship between light conditions and the development of symptoms. It is worth noting that the specific effects of light on plant virus symptoms can vary depending on the virus-host combination, light intensity, duration, and other environmental factors. The specific mechanism by which light affects the development of virus symptoms is still being investigated, but these findings highlight the importance of considering light conditions when studying plant-virus interactions and symptoms development.
3. The Role of Temperature in Virus-Induced Symptoms Development
Temperature is a crucial factor in determining the outcome of plant virus infections. However, the effects of increased temperatures on viral symptoms can vary depending on the specific virus-host combinations. Some studies have shown that higher temperatures can intensify virus symptoms [9], while in other cases, a phenomenon called “heat masking” occurs, where symptoms are reduced or eliminated despite the host remaining infected [10]. Recent research has described both positive [11][12] and negative [13][14][15][16] correlations between temperature and the severity of plant viral diseases. However, the underlying molecular mechanisms behind these phenomena are still not fully understood.
For example, in N. tabacum plants infected with CMV, higher incubation temperatures (28 °C compared to 18 °C) resulted in more severe symptoms. Molecular analysis of CMV-infected plants revealed that at lower temperatures, several genes associated with salicylic acid (SA) were upregulated, while at higher temperatures, genes associated with jasmonic acid (JA) showed increased expression [17]. SA-dependent responses are typically associated with plant defense against biotrophic pathogens, whereas JA pathways are often activated in response to necrotrophic pathogens. It is important to note that these two pathways, SA and JA, are antagonistic to each other. This means that when JA pathways are induced, plants become more susceptible to biotrophic pathogens, whereas the induction of SA pathways makes plants more vulnerable to necrotrophic pathogens.
At 25 °C, a severe strain of AltMV causes mosaic patterns and localized necrosis in N. benthamiana plants. However, when the temperature drops to 15 °C, these symptoms progress to systemic necrosis, resulting in plant death within 30 dpi. By contrast, a chimera consisting of the CP derived from a mild AltMV strain only induces systemic mosaic symptoms at 15 °C, reducing the severity of the symptoms caused by the severe strain. Surprisingly, there was no significant difference in virus accumulation between the severe and mild strains at a given temperature. However, it was observed that both strains exhibited significantly higher virus accumulation at 15 °C when compared to 25 °C [18]. This observation aligns with the trend of symptoms severity being correlated with the absolute titer of the symptoms-inducing factor. Further investigation revealed that the CP form responsible for inducing systemic necrosis interacts with a boron transporter. This interaction likely disrupts boron metabolism within the plant, ultimately triggering systemic necrosis.
These studies indicate that light and temperature can have diverse effects on plant viral infections and the severity of associated symptoms. The interplay between environmental factors, plant defense pathways, and viral factors contributes to the complex molecular mechanisms underlying these phenomena. Nevertheless, it is essential to note that beyond light and temperature, other environmental factors, such as CO2 concentration, UV radiation, ozone, and drought, can also influence the interactions between plant and virus [19][20][21][22]. Despite the progress made in this field, there is still a lack of comprehensive and in-depth studies, and a comprehensive understanding of these mechanisms and their implications for plant-virus interactions remains to be fully elucidated. Researchers need to conduct more comprehensive studies to gain a deeper insight into the impact of environmental factors on plant viral infections and how they affect the severity of symptoms.
This entry is adapted from the peer-reviewed paper 10.3390/plants12152830
References
- Harrison, B.D.; Jones, R.A.C. Effects of light and temperature on symptom development and virus content of tobacco leaves inoculated with potato mop-top virus. Ann. Appl. Biol. 1971, 67, 377.
- Osmond, C.B.; Berry, J.A.; Balachandran, S.; Buchenosmond, C.; Daley, P.F.; Hodgson, R.A.J. Potential consequences of virus-infection for shade-sun accumulation in leaves. Bot. Acta 1990, 103, 226–229.
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in plant-virus interaction. Front. Microbiol. 2016, 7, 1565.
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan horse in plant-virus interaction. Mol. Plant Pathol. 2018, 19, 504–518.
- Jiang, T.; Du, K.; Xie, J.; Sun, G.; Wang, P.; Chen, X.; Cao, Z.; Wang, B.; Chao, Q.; Li, X.; et al. Activated malate circulation contributes to the manifestation of light-dependent mosaic symptoms. Cell Rep. 2023, 42, 112333.
- Chen, Y.B.; Lu, T.C.; Wang, H.X.; Shen, J.; Bu, T.T.; Chao, Q.; Gao, Z.F.; Zhu, X.G.; Wang, Y.F.; Wang, B.C. Posttranslational modification of maize chloroplast pyruvate orthophosphate dikinase reveals the precise regulatory mechanism of its enzymatic activity. Plant Physiol. 2014, 165, 534–549.
- Chastain, C. Structure, Function, and Post-Translational Regulation of C4 Pyruvate Orthophosphate Dikinase; Raghavendra, A.S., Sage, R.F., Eds.; Springer Science + Business Media: Berlin, Germany, 2011; pp. 301–315.
- Ludwig, M. The roles of organic acids in C4 photosynthesis. Front. Plant Sci. 2016, 7, 647.
- Selman, I.W.; Grant, S.A. The influence of temperature and daylength on spotted wilt virus disease of tomato. Ann. Appl. Biol. 1957, 45, 312–317.
- Kassanis, B. Some effects of high temperature on the susceptibility of plants to infection with viruses. Ann. Appl. Biol. 1952, 39, 358–369.
- Chung, B.N.; Lee, J.H.; Kang, B.C.; Koh, S.W.; Joa, J.H.; Choi, K.S.; Ahn, J.J. HR-mediated defense response is overcome at high temperatures in capsicum species. Plant Pathol. J. 2018, 34, 71–77.
- Wosula, E.N.; Tatineni, S.; Wegulo, S.N.; Hein, G.L. Effect of temperature on wheat streak mosaic disease development in winter wheat. Plant Dis. 2017, 101, 324–330.
- Masinde, E.A.; Mkamillo, G.; Ogendo, J.O.; Hillocks, R.; Mulwa, R.M.S.; Kimata, B.; Maruthi, M.N. Genotype by environment interactions in identifying cassava (Manihot esculenta Crantz) resistant to cassava brown streak disease. Field Crops Res. 2018, 215, 39–48.
- Godfrey, S.; Settumba, M.; Samuel, K. Effect of temperature on sweet potato virus disease symptom expression. Afr. J. Agric. Res. 2017, 12, 2295–2309.
- del Toro, F.J.; Rakhshandehroo, F.; Larruy, B.; Aguilar, E.; Tenllado, F.; Canto, T. Effects of simultaneously elevated temperature and CO2 levels on Nicotiana benthamiana and its infection by different positive-sense RNA viruses are cumulative and virus type-specific. Virology 2017, 511, 184–192.
- Chung, B.N.; Canto, T.; Tenllado, F.; Choi, K.S.; Joa, J.H.; Ahn, J.J.; Kim, C.H.; Do, K.S. The effects of high temperature on infection by potato virus Y, potato virus A, and potato leafroll virus. Plant Pathol. J. 2016, 32, 321–328.
- Zhao, F.; Li, Y.; Chen, L.; Zhu, L.; Ren, H.; Lin, H.; Xi, D. Temperature dependent defence of Nicotiana tabacum against Cucumber mosaic virus and recovery occurs with the formation of dark green islands. J. Plant Biol. 2016, 59, 293–301.
- Lim, H.S.; Nam, J.; Seo, E.Y.; Nam, M.; Vaira, A.M.; Bae, H.; Jang, C.Y.; Lee, C.H.; Kim, H.G.; Roh, M.; et al. The coat protein of Alternanthera mosaic virus is the elicitor of a temperature-sensitive systemic necrosis in Nicotiana benthamiana, and interacts with a host boron transporter protein. Virology 2014, 452–453, 264–278.
- Vasquez, D.F.; Hernandez, A.; Torres, D.; Borrero-Echeverry, F.; Zuluaga, P.; Rincon, D.F. Drought as a modulator of plant–virus–vector interactions: Effects on symptom expression, plant immunity and vector behaviour. Plant Pathol. 2022, 71, 1282–1292.
- Prasad, A.; Sett, S.; Prasad, M. Plant-virus-abiotic stress interactions: A complex interplay. Environ. Exp. Bot. 2022, 199, 104869.
- Bilgin, D.D.; Aldea, M.; O’Neill, B.F.; Benitez, M.; Li, M.; Clough, S.J.; DeLucia, E.H. Elevated ozone alters soybean-virus interaction. Mol. Plant Microbe Interact. 2008, 21, 1297–1308.
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet radiation from a plant perspective: The plant-microorganism context. Front. Plant Sci. 2020, 11, 597642.
This entry is offline, you can click here to edit this entry!
