Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Bariatric surgery has been shown to be able to reduce the incidence of obesity-related cardiovascular disease and thus overall mortality. This result has been shown to be the result of hormonal and metabolic effects induced by post-surgical anatomical changes, with important effects on multiple hormonal and molecular axes that make this treatment more effective than conservative therapy in determining a marked improvement in the patient’s cardiovascular risk profile.
- bariatric surgery
- obesity
- diabetes mellitus
- cardiovascular disease
1. Introduction
Obesity is a chronic disease, characterized by an excess of adipose tissue, whose etiology is complex and multifactorial, resulting from the interaction of numerous genetic and environmental factors [1]. According to the World Health Organization, the global prevalence of obesity has more than doubled since 1980, reaching a pandemic proportion, for which the term “globesity” has been coined [2]. It is estimated that up to 35% of the world’s population has problems with excess weight [1]. The phenomenon is growing rapidly and also affecting the young (children and adolescents), with significant social costs [3]. In Europe, up to 59% of adults and almost 1 in 3 children are overweight or affected by obesity. Moreover, it is one of the main causes of death in the European Region, with more than 1.2 million deaths per year (corresponding to more than 13% of total mortality) [4].
The pathophysiological mechanisms linking obesity and the risk of cardiovascular diseases (CVDs) and adverse cardiovascular events are multiple and different [5]. Obesity induces insulin resistance and consequent hyperinsulinemia [6], increases the basal sympathetic tone [7] and the excess adipose tissue produces a series of molecules, called “adipokines” [8], which promote systemic inflammation and induce a pro-thrombotic state [9][10][11], all conditions known to be associated with the risk of coronary artery diseases development and progression. Obesity is a real disease that reduces the quality and life expectancy, and taking into account the molecular mechanisms described above, it represents a risk factor for the onset of diseases such as hypertension (HTN), type 2 diabetes mellitus (T2DM), sleep apnea [12] and metabolic syndrome, leading to myocardial infarction (MI) and stroke [5].
The presence of obesity worsens glycemic control and metabolic parameters, thus inducing diabetes. It is known that the risk of T2DM increases linearly with body mass index (BMI) [13]. Because of this strict relationship, the term “diabesity” is also used [14]. For the management of this condition, lifestyle changes are mandatory in addition to innovative and effective pharmacological and surgical strategies [1]. It is important to personalize the hypoglycemic therapy according to the patient’s phenotype, considering in particular those with a neutral or weight-reducing effect. Bariatric surgical procedures (BS) decrease the CVD risk, leading to sustained weight loss and improvement of glycemic control, HTN, and dyslipidemia in patients with T2DM, also after many years from the intervention [15] and have been found superior compared to those who did not undergo surgical treatment [16]. This surgery, also called “metabolic”, allows better control of diabetes through reduction of caloric intake, weight loss, and reduction of insulin resistance. T2DM is considered a criterion for bariatric surgery in patients with BMI > 35 kg/m2 [17] and several studies have shown that bariatric surgery leads to decreased long-term mortality by improving CVD risk profile [18][19][20][21] and reducing the risk of MI as well [22].
2. Bariatric Surgery
Bariatric surgery refers to a group of gastrointestinal surgeries whose original aim was to achieve weight loss in extremely obese populations. However, since its introduction in the 1950s [23], it has been observed that in patients undergoing such surgery there was a drastic reduction in obesity-associated diseases including primarily T2DM [24] but also HTN, dyslipidemia, and overall mortality [25][26]. To emphasize the clinical importance of these beneficial systemic effects, these procedures are now better defined under the term metabolic bariatric surgery (MBS) [27]. Accumulated evidence in the last few years has demonstrated a significant variation in the secretion and activity of hormones and neurotransmitters. Some of these mediators are able to affect appetite and satiety as well as energy expenditure and glucose metabolism, making it clear that the post-surgical metabolic effects were not exclusively related to the mere weight loss (caloric restriction) and malabsorption that follow such surgical procedures [28][29].
Currently, MBS are categorized by their predominant mechanism of action in pure gastric restrictive procedures (adjustable gastric band, sleeve gastrectomy), gastric restriction with significant malabsorption (malabsorptive surgeries: biliopancreatic diversion and duodenal switch), and gastric restriction with poor malabsorption (mixed restrictive and malabsorptive surgeries: Roux-en-Y gastric bypass and one-anastomosis gastric bypass) [30]. The jejunocolic bypass was first performed in 1970; it is an example of a pure malabsorptive procedure, and was abandoned due to severe side effects [31].
Pure gastric restrictive interventions are procedures that result in a reduction of gastric capability by the creation of a smaller gastric chamber called a “pouch”, leading to weight loss because of early satiety during food intake, thus associating to a lower intake of calories. Among them, adjustable gastric banding (AGB) and sleeve gastrectomy (SG) are the most performed procedures (Figure 1) [32].
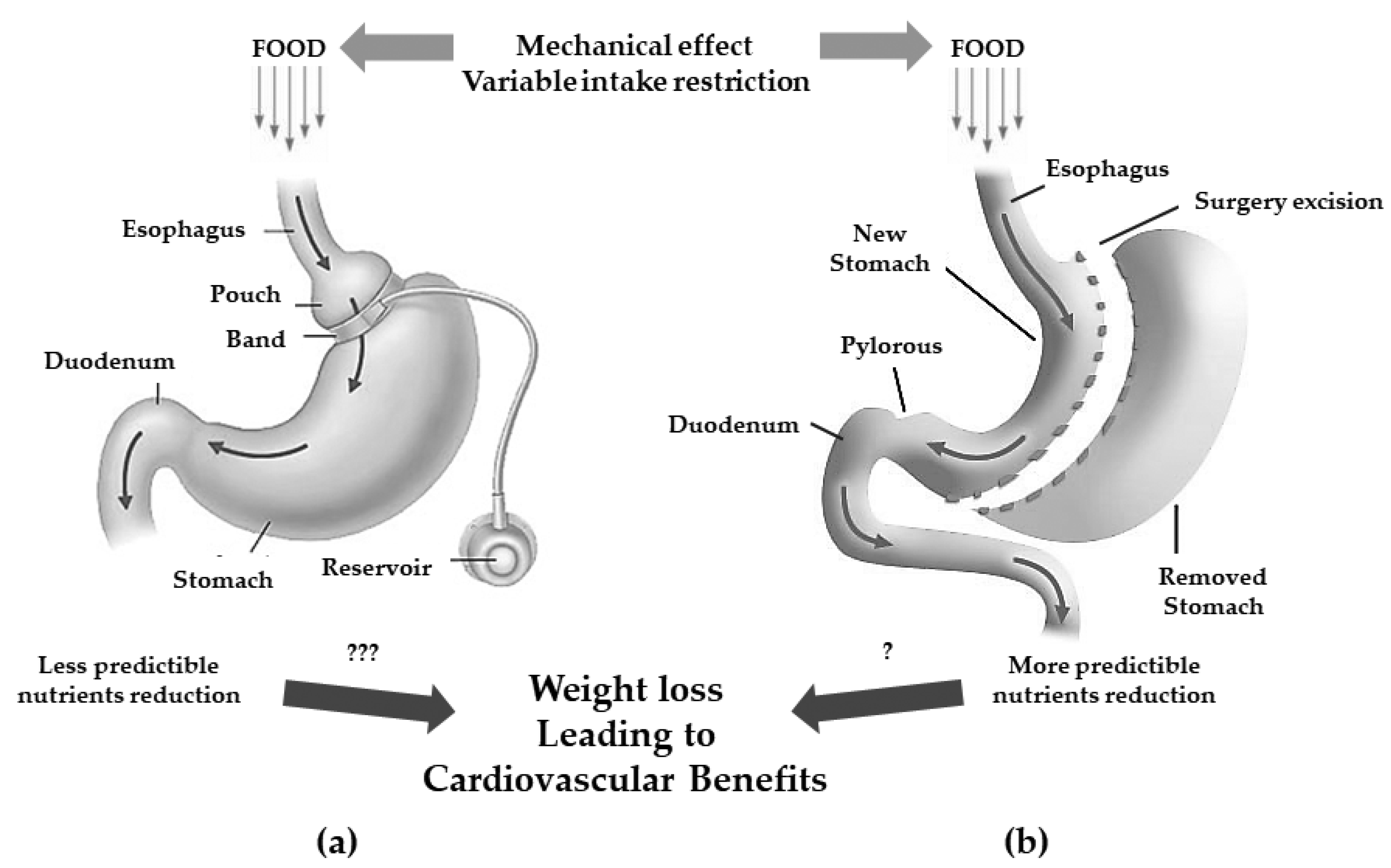
Figure 1. Gastric restrictive bariatric surgeries. (a): adjustable gastric band with less predictibale nutrients reduction (???); (b): sleeve gastrectomy with more predictable nutrients reduction (?) and possible cardiovascular benefits.
The AGB consists of a laparoscopic positioning of a silicone prosthesis (the band) around the stomach creating a proximal gastric pouch of approximately 20–30 mL. The prosthesis is adjustable, i.e., it has the possibility of tightening or widening the passage between the pouch above and the remaining stomach (outlet) below the banding; this occurs because the banding consists of an insufflation chamber that is connected to a valve, positioned in the subcutaneous tissue, through a catheter [33].
The SG consists of the vertical partition of the stomach reaching a reduction of its size of about 25%.
Originally, the SG was the first step to execute either a gastric by-pass (GPB) or biliopancreatic diversion (BPD) with duodenal switch (DS) but since most patients showed to successfully achieve satisfactory weight loss without the full procedure, it is now a standing-alone surgery which trend is constantly increasing [32]. Even if it is commonly considered a restrictive procedure, it has been demonstrated that the SG can induce several hormonal changes turning its mechanism of action more complex and useful to determine also metabolic changes [34].
Currently, the two most frequent malabsorptive procedures performed worldwide are BPD and DS [35] (Figure 2).
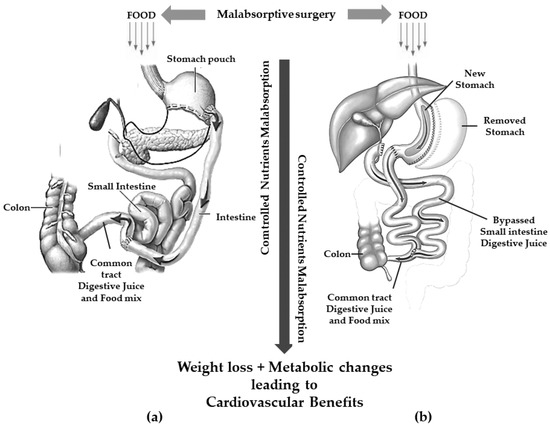
Figure 2. Gastric restriction procedures with significant malabsorption. (a): biliopancreatic diversion; (b): duodenal switch.
These procedures are thought to achieve weight loss through a controlled malabsorption of nutrients.
BPD consists of the resection of approximately two distal thirds of the stomach with the closure of the duodenal stump followed by an intestinal bypass. The procedure allows the creation of two tubes: food passes through one and biliopancreatic secretions from the liver and pancreas pass through the other. The meeting of pancreatic secretion and food only takes place for a short distance, approximately 70 cm from the colon, thus resulting in reduced digestion and less food absorption [36]. The gastric pouch is larger than those of the restrictive procedures allowing larger meals even compared to GBP [30].
To avoid or reduce several complications related to BPD (mainly dumping syndrome and marginal ulcer), a SG is executed instead of a distal gastric resection in the so-called BPD/DS [37].
Roux-en-Y gastric bypass (RYGB) is a mixed restrictive and malabsorptive procedure. Weight loss is achieved through gastric restriction and decreased intestinal absorption, which is the greater the further downstream the outlet of the bilio-pancreatic secretions. It consists of the definitive separation of the stomach with the creation of a small gastric pouch of 25–30 mL. This pouch is anastomosed with the alimentary tract of a digiunal loop, while the biliopancreatic tract is anastomosed between 100 and 150 cm downstream of the gastrodigiunal anastomosis [38].
Today, one-anastomosis gastric bypass (OAGB) is the third most commonly performed MBS surgery. It is a modification of the mini-gastric bypass that originated in 1997 [39] and now counts several technical variants [40] but overall, it consists of a long narrow-sleeve gastric tube in conjunction with end-to-side or side-to-side gastrojejunostomy performed 150–200 cm distal to the ligament of Treitz [41][42]. A schematic view of RYGB and OAGB is shown in Figure 3.
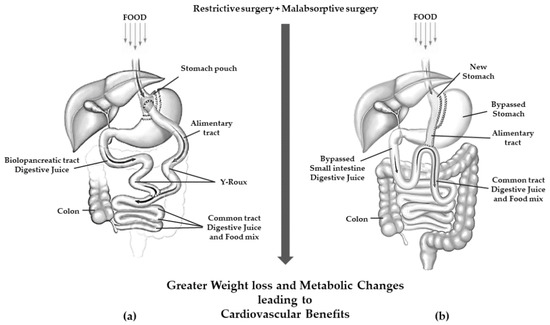
Figure 3. Gastric resection with poor malabsorption surgery (mixed restrictive and ma absorptive surgery): (a) Roux en-Y gastric bypass; (b) one anastomosis gastric bypass.
OAGB has been associated with a greater weight loss than RYGB [43]. Furthermore, in patients with very high BMI (≥60 kg/m2), OAGB was shown to be non-inferior to RYGB with regard to weight loss in a 2 years follow-up [44].
There are no absolute contraindications to BS but severe heart failure, unstable coronary artery disease, end-stage lung disease, active cancer treatment, portal hypertension, drug/alcohol dependency, and impaired intellectual capacity are considered relative contraindications [30].
3. Pathophysiological Changes after Bariatric Surgery
Bariatric surgery reduces the incidence of CVD through multiple hormonal mechanisms [45][46][47][48]. Because the residual volume of the stomach limits food intake, it is mandatory a change in eating habits. The 2014 European guidelines suggest a small-fractionated meal plan for all patients undergoing bariatric surgery [49]. These changes in eating style may affect hormone signaling in the liver such as insulin, glucagon, ghrelin, and many others [50].
The primary change after BS is weight loss, which results in several benefits [51]. A previous study has evaluated how different degrees of weight loss may affect metabolic function and adipose tissue biology [52]. A weight loss greater than 16 percent of initial weight results in a significant decrease in free fatty acids (FFA) and C-reactive protein (CRP) plasma concentrations [53][54]. Conversely, plasma adiponectin levels raised significantly [55], and a preferential loss of intra-abdominal and intrahepatic fat compared to total body fat was also found [52]. Hepatic benefits correlate with the degree of weight loss: a decrease of 3–5% is associated with reduced steatosis, ≥5–7% resolved NASH while greater reductions (i.e., ≥10%) may also improve liver fibrosis [56]. Weight loss is also associated with histologic improvement and this effect is directly correlated to the degree of weight reduction regardless of the method used to achieve it [57].
After bariatric surgery, blood levels of triglycerides and glucose are significantly reduced, while postprandial levels of adiponectin, glucagon-like peptide 1 (GLP-1), insulin, and serum insulin-like growth factor-1 (IGF-1) are significantly increased. Elevated adiponectin levels are associated with changes in total fat mass and reduced risk of atherosclerosis [58]. Increased GLP-1 levels after weight loss surgery can improve obesity-induced endothelial dysfunction, restore the endothelial protective properties of high-density lipoprotein cholesterol (HDL-C) [59], and reduce insulin resistance. These metabolic changes result consequently in a decreased incidence of common carotid artery intima-media thickness augmentation especially in young patients with morbid obesity [5]. Serum Hsp60 is increased in morbidly obese patients and decreases after post-surgical weight loss. The association of Hsp60 with cardiovascular risk as a pro-inflammatory adipofactor suggests that Hsp60 may be a potential link between adipose tissue inflammation and CVD development [60].
Weight loss after bariatric surgery may help blood pressure control in obese patients. Previous observations have shown a remission rate of HTN between 60% and 70% in the year following weight loss surgery reaching a peak as high as 90% in a medium follow-up [61][62].
In this regard, the prospective, observational, unicenter BARIHTA study (Hemodynamic Changes And Vascular Tone Control After BARIatric Surgery. Prognostic Value Regarding HyperTension And Target Organ Damage), has reported that patients with severe obesity scheduled to undergo bariatric surgery [63] showed a statistically significant decrease in plasmatic renin activity (PRA), aldosterone, angiotensin-converting enzyme (ACE) activity with an increase in the ACE/ACE2 ratio [63].
Bariatric surgery also reduces the rates of T2DM because of better glucose control that may lead to a remission of diabetes in up to 95–100% of patients [64]. Initially, weight loss was thought to be the major determinant of this benefit [65]. However, different studies have found that metabolic changes and modulation of the hormones profile play a greater role, in improving incretin secretion, recovering islet function, and restoring peripheral insulin sensitivity to regulate glucose homeostasis [66][67][68][69][70][71][72].
Weight loss after bariatric surgery modulates GLP-1 levels [73][74][75][76]. This mediator plays an important role in changes in metabolism [74]. GLP-1 is a peptide hormone and neurotransmitter with several metabolic and non-metabolic effects such as its ability to improve B-cell function and insulin sensitivity [71][75][76][77]. The rapid entry and absorption of nutrients in the distal small intestine induce increases in GLP-1 (up to three-fold). Although the majority of GLP-1 research has focused on glycemic control, GLP-1 functions extend beyond glucose metabolism. It is known that GLP-1 has a dose-dependent effect on satiety [74].
Peptide YY (PYY) is a peptide released by enteroendocrine L cells in the distal small intestine and colon in response to feeding [78]. It is mainly involved in the central regulation of appetite [79]. It has been shown in an experimental model that PYY mediated weight loss after bypass surgery [80]. Moreover, increased PYY has been observed in patients after bariatric surgery [81]. PYY is also believed to regulate glucose homeostasis [82]. Although further studies are needed, it is plausible that increased PYY levels are associated with the stabilization of glucose levels, metabolism, and weight, which have a direct impact on both the rates and complications of obesity and diabetes.
Although patients with T2DM openly outnumber patients with T1DM, bariatric surgery has been associated also with benefits on T1DM subjects and associated biomarkers. One study indicated that comparable benefits might be achieved by bariatric surgery on complications associated with T1DM as well as T2DM [73].
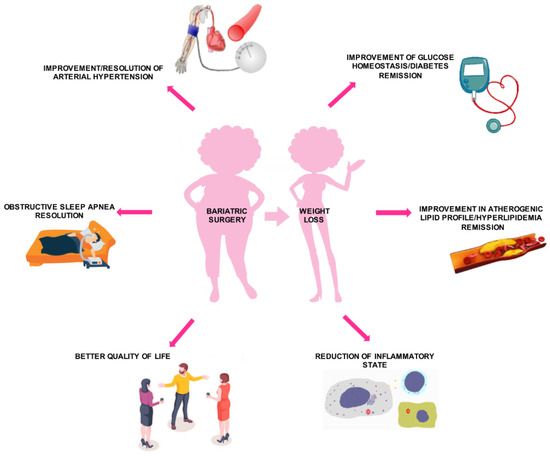
4. Effects of Bariatric Surgery on Diabetes Mellitus and Other Cardiovascular Risk Factors
Bariatric surgery has been proven to achieve benefic results on cardiovascular risk, by short- and long-term effects on diabetes mellitus, HTN, dyslipidemia, and inflammation, leading to a better quality of life (Figure 4).

Figure 4. Effects of bariatric surgery on cardiovascular risk profile.
4.1. T2DM
Several, large cohort studies comparing BS to conventional obesity management have confirmed that patients undergoing bariatric surgery achieve diabetes remission more frequently than those on conventional therapy alone, over a follow-up period ranging from one to 5 years [83][84][85][86].
Glycemic profile improvement after bariatric surgery is largely due to the weight loss and the subsequent increase in insulin sensitivity, as it happens to patients who lose an equivalent amount of weight by using caloric restriction [87]. A substantial improvement in the glycemic balance is also determined by changes in the gut microbiome, gut hormones, and bile acid signaling, which typically occur early after bariatric surgery.
Within 3 months from surgery, the gut microbiome appears markedly altered, with increased diversity [88][89][90][91]. In support of the above, fecal transplant from either mice or humans that have undergone RYGB into germ-free rats fed on a high-fat diet results in weight loss and improvement in glycemic parameters [92].
4.2. Systemic Arterial HTN
Bariatric surgery leads to improvement or resolution of HTN. A systematic review and meta-analysis of prospective studies have shown that a BMI reduction of 5 corresponds to an HTN reduction of 27% after 12–24 months from surgery [93].
Decrease in vasoconstrictors (angiotensinogen, angiotensin II, renin, and endothelin-1), increase in vasodilators, and natriuresis (induced by higher levels of atrial natriuretic peptide, due to neprylisin reduction) are key factors which mediate blood pressure reduction [94][95].
HTN improvement after bariatric surgery is also due to obstructive sleep apnea resolution. Obstructive sleep apnea syndrome in obese people is characterized by intermittent hypoxia/hypercapnia, which causes the activation of the sympathetic nervous system and renin-angiotensin-aldosterone system, contributing significantly to HTN
4.3. Dyslipidemia
Bariatric surgery-induced weight loss results in significant improvement of atherogenic lipid profile, in terms of reduction in total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides, and increase in cardioprotective HDL-C [96][97]. However, the magnitude of these changes varies widely among the different bariatric surgical techniques, likely due to anatomic alterations unique to each procedure [97][98].
4.4. Inflammation
Bariatric surgery has been proven to reduce obesity-related inflammatory states. A meta-analysis of 52 studies reported a decrease in serum inflammatory markers such as CRP levels, measured by high-sensitivity assay (hs-CRP), of 61.7%, up to 34 months after bariatric surgery [99].
4.5. Health-Related Quality of Life
The amelioration or resolution of comorbidities typically associated with obesity, including T2DM, HTN, obstructive sleep apneas, dyslipidemia, and gastroesophageal reflux disease, after bariatric surgery, improves significantly patients’ quality of life.
This entry is adapted from the peer-reviewed paper 10.3390/life13071552
References
- Clark, J.M.; Garvey, W.T.; Niswender, K.D.; Schmidt, A.M.; Ahima, R.S.; Aleman, J.O.; Battarbee, A.N.; Beckman, J.; Bennett, W.L.; Brown, N.J.; et al. Obesity and Overweight: Probing Causes, Consequences, and Novel Therapeutic Approaches Through the American Heart Association’s Strategically Focused Research Network. J. Am. Heart Assoc. 2023, 12, e027693.
- The Lancet Gastroenterology & Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411.
- Santos, F.G.C.D.; Godoy-Leite, M.; Penido, E.A.R.; Ribeiro, K.A.; da Gloria Rodrigues-Machado, M.; Rezende, B.A. Eating behaviour, quality of life and cardiovascular risk in obese and overweight children and adolescents: A cross-sectional study. BMC Pediatr. 2023, 23, 299.
- Krzysztoszek, J.; Laudańska-Krzemińska, I.; Bronikowski, M. Assessment of epidemiological obesity among adults in EU countries. Ann. Agric. Environ. Med. 2019, 26, 341–349.
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010.
- Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov. Today 2022, 27, 822–830.
- Lambert, G.W.; Schlaich, M.P.; Eikelis, N.; Lambert, E.A. Sympathetic activity in obesity: A brief review of methods and supportive data. Ann. N. Y. Acad. Sci. 2019, 1454, 56–67.
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564.
- Cirillo, P.; Ziviello, F.; Pellegrino, G.; Conte, S.; Cimmino, G.; Giaquinto, A.; Pacifico, F.; Leonardi, A.; Golino, P.; Trimarco, B. The adipokine apelin-13 induces expression of prothrombotic tissue factor. Thromb. Haemost. 2015, 113, 363–372.
- Cirillo, P.; Di Palma, V.; Maresca, F.; Pacifico, F.; Ziviello, F.; Bevilacqua, M.; Trimarco, B.; Leonardi, A.; Chiariello, M. The adipokine visfatin induces tissue factor expression in human coronary artery endothelial cells: Another piece in the adipokines puzzle. Thromb. Res. 2012, 130, 403–408.
- Calabro, P.; Cirillo, P.; Limongelli, G.; Maddaloni, V.; Riegler, L.; Palmieri, R.; Pacileo, G.; De Rosa, S.; Pacileo, M.; De Palma, R.; et al. Tissue factor is induced by resistin in human coronary artery endothelial cells by the NF-kB-dependent pathway. J. Vasc. Res. 2011, 48, 59–66.
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88.
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20.
- Ng, A.C.T.; Delgado, V.; Borlaug, B.A.; Bax, J.J. Diabesity: The combined burden of obesity and diabetes on heart disease and the role of imaging. Nat. Rev. Cardiol. 2020, 18, 291–304.
- Rubio-Almanza, M.; Camara-Gomez, R.; Hervas-Marin, D.; Ponce-Marco, J.L.; Merino-Torres, J.F. Cardiovascular risk reduction over time in patients with diabetes or pre-diabetes undergoing bariatric surgery: Data from a single-center retrospective observational study. BMC Endocr. Disord. 2018, 18, 90.
- McGrice, M.; Don Paul, K. Interventions to improve long-term weight loss in patients following bariatric surgery: Challenges and solutions. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 263–274.
- 2004 ASBS Consensus Conference on Surgery for Severe Obesity. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2005, 1, 297–381.
- Coutinho, T.; Goel, K.; Correa de Sa, D.; Carter, R.E.; Hodge, D.O.; Kragelund, C.; Kanaya, A.M.; Zeller, M.; Park, J.S.; Kober, L.; et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “normal weight central obesity”. J. Am. Coll. Cardiol. 2013, 61, 553–560.
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489.
- Maessen, D.E.; Stehouwer, C.D.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861.
- Kim, J.; Eisenberg, D.; Azagury, D.; Rogers, A.; Campos, G.M. American Society for Metabolic and Bariatric Surgery position statement on long-term survival benefit after metabolic and bariatric surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2016, 12, 453–459.
- Kwok, C.S.; Pradhan, A.; Khan, M.A.; Anderson, S.G.; Keavney, B.D.; Myint, P.K.; Mamas, M.A.; Loke, Y.K. Bariatric surgery and its impact on cardiovascular disease and mortality: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 20–28.
- Henrikson, V. Can Small Bowel Resection Be Defended as Therapy for Obesity? Obes. Surg. 1994, 4, 54.
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, A.; Bouchard, C.; Carlsson, B.; Karason, K.; Lonroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304.
- Zhou, X.; Yu, J.; Li, L.; Gloy, V.L.; Nordmann, A.; Tiboni, M.; Li, Y.; Sun, X. Effects of Bariatric Surgery on Mortality, Cardiovascular Events, and Cancer Outcomes in Obese Patients: Systematic Review and Meta-analysis. Obes. Surg. 2016, 26, 2590–2601.
- Benotti, P.N.; Wood, G.C.; Carey, D.J.; Mehra, V.C.; Mirshahi, T.; Lent, M.R.; Petrick, A.T.; Still, C.; Gerhard, G.S.; Hirsch, A.G. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. J. Am. Heart Assoc. 2017, 6, e005126.
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2022, 18, 1345–1356.
- Heneghan, H.M.; Nissen, S.; Schauer, P.R. Gastrointestinal surgery for obesity and diabetes: Weight loss and control of hyperglycemia. Curr. Atheroscler. Rep. 2012, 14, 579–587.
- Miras, A.D.; le Roux, C.W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584.
- Jaunoo, S.S.; Southall, P.J. Bariatric surgery. Int. J. Surg. 2010, 8, 86–89.
- Baker, M.T. The history and evolution of bariatric surgical procedures. Surg. Clin. N. Am. 2011, 91, 1181–1201.
- Phillips, B.T.; Shikora, S.A. The history of metabolic and bariatric surgery: Development of standards for patient safety and efficacy. Metab. Clin. Exp. 2018, 79, 97–107.
- Furbetta, N.; Cervelli, R.; Furbetta, F. Laparoscopic adjustable gastric banding, the past, the present and the future. Ann. Transl. Med. 2020, 8, S4.
- Karamanakos, S.N.; Vagenas, K.; Kalfarentzos, F.; Alexandrides, T.K. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: A prospective, double blind study. Ann. Surg. 2008, 247, 401–407.
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Zundel, N.; Buchwald, H.; Scopinaro, N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes. Surg. 2017, 27, 2279–2289.
- Scopinaro, N.; Marinari, G.M.; Camerini, G. Laparoscopic standard biliopancreatic diversion: Technique and preliminary results. Obes. Surg. 2002, 12, 241–244.
- Biertho, L.; Lebel, S.; Marceau, S.; Hould, F.S.; Julien, F.; Biron, S. Biliopancreatic Diversion with Duodenal Switch: Surgical Technique and Perioperative Care. Surg. Clin. N. Am. 2016, 96, 815–826.
- Schauer, P.R. Open and laparoscopic surgical modalities for the management of obesity. J. Gastrointest. Surg. 2003, 7, 468–475.
- Rutledge, R. The mini-gastric bypass: Experience with the first 1,274 cases. Obes. Surg. 2001, 11, 276–280.
- Mahawar, K.K.; Kular, K.S.; Parmar, C.; Van den Bossche, M.; Graham, Y.; Carr, W.R.J.; Madhok, B.; Magee, C.; Purkayastha, S.; Small, P.K. Perioperative Practices Concerning One Anastomosis (Mini) Gastric Bypass: A Survey of 210 Surgeons. Obes. Surg. 2018, 28, 204–211.
- Ramos, A.C.; Chevallier, J.M.; Mahawar, K.; Brown, W.; Kow, L.; White, K.P.; Shikora, S.; Contributors, I.C.C. IFSO (International Federation for Surgery of Obesity and Metabolic Disorders) Consensus Conference Statement on One-Anastomosis Gastric Bypass (OAGB-MGB): Results of a Modified Delphi Study. Obes. Surg. 2020, 30, 1625–1634.
- Deitel, M. Consensus survey on mini-gastric bypass and one-anastomosis gastric bypass. Ann. Bariatr. Metab. Surg. 2018, 1, 1001.
- Bhandari, M.; Nautiyal, H.K.; Kosta, S.; Mathur, W.; Fobi, M. Comparison of one-anastomosis gastric bypass and Roux-en-Y gastric bypass for treatment of obesity: A 5-year study. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2019, 15, 2038–2044.
- Parmar, C.; Abdelhalim, M.A.; Mahawar, K.K.; Boyle, M.; Carr, W.R.J.; Jennings, N.; Small, P.K. Management of super-super obese patients: Comparison between one anastomosis (mini) gastric bypass and Roux-en-Y gastric bypass. Surg. Endosc. 2017, 31, 3504–3509.
- Fisher, D.P.; Johnson, E.; Haneuse, S.; Arterburn, D.; Coleman, K.J.; O’Connor, P.J.; O’Brien, R.; Bogart, A.; Theis, M.K.; Anau, J.; et al. Association Between Bariatric Surgery and Macrovascular Disease Outcomes in Patients With Type 2 Diabetes and Severe Obesity. JAMA 2018, 320, 1570–1582.
- Doumouras, A.G.; Wong, J.A.; Paterson, J.M.; Lee, Y.; Sivapathasundaram, B.; Tarride, J.E.; Thabane, L.; Hong, D.; Yusuf, S.; Anvari, M. Bariatric Surgery and Cardiovascular Outcomes in Patients With Obesity and Cardiovascular Disease:: A Population-Based Retrospective Cohort Study. Circulation 2021, 143, 1468–1480.
- Domenech-Ximenos, B.; Cuba, V.; Daunis, I.E.P.; Thio-Henestrosa, S.; Jaldo, F.; Biarnes, C.; Molina, X.; Xifra, G.; Ricart, W.; Bardera, A.; et al. Bariatric Surgery-Induced Changes in Intima-Media Thickness and Cardiovascular Risk Factors in Class 3 Obesity: A 3-Year Follow-Up Study. Obesity 2020, 28, 1663–1670.
- Aminian, A.; Aleassa, E.M.; Bhatt, D.L.; Tu, C.; Khorgami, Z.; Schauer, P.R.; Brethauer, S.A.; Daigle, C.R. Bariatric surgery is associated with a lower rate of death after myocardial infarction and stroke: A nationwide study. Diabetes Obes. Metab. 2019, 21, 2058–2067.
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Fruhbeck, G.; International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55.
- Tong, X.; Yin, L. Circadian rhythms in liver physiology and liver diseases. Compr. Physiol. 2013, 3, 917–940.
- Reynolds, E.L.; Watanabe, M.; Banerjee, M.; Chant, E.; Villegas-Umana, E.; Elafros, M.A.; Gardner, T.W.; Pop-Busui, R.; Pennathur, S.; Feldman, E.L.; et al. The effect of surgical weight loss on diabetes complications in individuals with class II/III obesity. Diabetologia 2023, 66, 1192–1207.
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601.
- Selvin, E.; Paynter, N.P.; Erlinger, T.P. The effect of weight loss on C-reactive protein: A systematic review. Arch. Intern. Med. 2007, 167, 31–39.
- Jia, Q.; Carranza Leon, B.G.; Jensen, M.D. Influence of Free Fatty Acid Concentrations and Weight Loss on Adipose Tissue Direct Free Fatty Acid Storage Rates. J. Clin. Endocrinol. Metab. 2021, 106, e5165–e5179.
- Wooten, J.S.; Breden, M.; Hoeg, T.; Smith, B.K. Effects of weight-loss on adipokines, total and regional body composition and markers of metabolic syndrome in women who are overweight and obese. Endocr. Metab. Sci. 2022, 7–8, 100120.
- Hannah, W.N., Jr.; Harrison, S.A. Lifestyle and Dietary Interventions in the Management of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1365–1374.
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, S.M.; Patterson, B.W.; Klein, S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009, 136, 1552–1560.
- Umeda, L.M.; Pereira, A.Z.; Carneiro, G.; Arasaki, C.H.; Zanella, M.T. Postprandial adiponectin levels are associated with improvements in postprandial triglycerides after Roux-en-Y gastric bypass in type 2 diabetic patients. Metab. Syndr. Relat. Disord. 2013, 11, 343–348.
- Osto, E.; Doytcheva, P.; Corteville, C.; Bueter, M.; Dorig, C.; Stivala, S.; Buhmann, H.; Colin, S.; Rohrer, L.; Hasballa, R.; et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: Role of glucagon-like peptide-1. Circulation 2015, 131, 871–881.
- Sell, H.; Poitou, C.; Habich, C.; Bouillot, J.L.; Eckel, J.; Clement, K. Heat Shock Protein 60 in Obesity: Effect of Bariatric Surgery and its Relation to Inflammation and Cardiovascular Risk. Obesity 2017, 25, 2108–2114.
- Benaiges, D.; Climent, E.; Goday, A.; Flores-Le Roux, J.A.; Pedro-Botet, J. Bariatric surgery and hypertension: Implications and perspectives after the GATEWAY randomized trial. Cardiovasc. Diagn. Ther. 2019, 9, 100–103.
- Jakobsen, G.S.; Smastuen, M.C.; Sandbu, R.; Nordstrand, N.; Hofso, D.; Lindberg, M.; Hertel, J.K.; Hjelmesaeth, J. Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. JAMA 2018, 319, 291–301.
- Oliveras, A.; Molina, L.; Goday, A.; Sans, L.; Riera, M.; Vazquez, S.; Benaiges, D.; Granados, A.M.; Ramon, J.M.; Pascual, J. Effect of bariatric surgery on cardiac structure and function in obese patients: Role of the renin-angiotensin system. J. Clin. Hypertens. 2021, 23, 181–192.
- Stefater, M.A.; Inge, T.H. Bariatric Surgery for Adolescents with Type 2 Diabetes: An Emerging Therapeutic Strategy. Curr. Diabetes Rep. 2017, 17, 62.
- Schauer, P.R.; Nor Hanipah, Z.; Rubino, F. Metabolic surgery for treating type 2 diabetes mellitus: Now supported by the world’s leading diabetes organizations. Clevel. Clin. J. Med. 2017, 84, S47–S56.
- Hankir, M.K.; Rullmann, M.; Seyfried, F.; Preusser, S.; Poppitz, S.; Heba, S.; Gousias, K.; Hoyer, J.; Schutz, T.; Dietrich, A.; et al. Roux-en-Y gastric bypass surgery progressively alters radiologic measures of hypothalamic inflammation in obese patients. JCI Insight 2019, 4, e131329.
- Cerit, Z. Bariatric surgery, diabetes mellitus, and epicardial adipose tissue. Nutr. Metab. Cardiovasc. Dis. NMCD 2017, 27, 581.
- Buchwald, H.; Buchwald, J.N. Metabolic (Bariatric and Nonbariatric) Surgery for Type 2 Diabetes: A Personal Perspective Review. Diabetes Care 2019, 42, 331–340.
- Argyropoulos, G. Bariatric surgery: Prevalence, predictors, and mechanisms of diabetes remission. Curr. Diabetes Rep. 2015, 15, 15.
- Torquati, A.; Shantavasinkul, P.C.; Omotosho, P.; Corsino, L.; Spagnoli, A. Perioperative changes in prouroguanylin hormone response in severely obese subjects after bariatric surgery. Surgery 2019, 166, 456–459.
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364.
- Guerrero-Perez, F.; Casajoana, A.; Gomez-Vaquero, C.; Virgili, N.; Lopez-Urdiales, R.; Hernandez-Montoliu, L.; Pujol-Gebelli, J.; Osorio, J.; Alves, C.; Perez-Maraver, M.; et al. Changes in Bone Mineral Density in Patients with Type 2 Diabetes After Different Bariatric Surgery Procedures and the Role of Gastrointestinal Hormones. Obes. Surg. 2020, 30, 180–188.
- Ji, Y.; Lee, H.; Kaura, S.; Yip, J.; Sun, H.; Guan, L.; Han, W.; Ding, Y. Effect of Bariatric Surgery on Metabolic Diseases and Underlying Mechanisms. Biomolecules 2021, 11, 1582.
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsboll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403.
- Jimenez, A.; Mari, A.; Casamitjana, R.; Lacy, A.; Ferrannini, E.; Vidal, J. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes 2014, 63, 3372–3377.
- Jimenez, A.; Casamitjana, R.; Viaplana-Masclans, J.; Lacy, A.; Vidal, J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013, 36, 2062–2069.
- Jorgensen, N.B.; Jacobsen, S.H.; Dirksen, C.; Bojsen-Moller, K.N.; Naver, L.; Hvolris, L.; Clausen, T.R.; Wulff, B.S.; Worm, D.; Lindqvist Hansen, D.; et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E122–E131.
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654.
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003, 349, 941–948.
- Chandarana, K.; Gelegen, C.; Karra, E.; Choudhury, A.I.; Drew, M.E.; Fauveau, V.; Viollet, B.; Andreelli, F.; Withers, D.J.; Batterham, R.L. Diet and gastrointestinal bypass-induced weight loss: The roles of ghrelin and peptide YY. Diabetes 2011, 60, 810–818.
- Magouliotis, D.E.; Tasiopoulou, V.S.; Sioka, E.; Chatedaki, C.; Zacharoulis, D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: A Systematic Review and Meta-analysis. Obes. Surg. 2017, 27, 1345–1357.
- Boey, D.; Sainsbury, A.; Herzog, H. The role of peptide YY in regulating glucose homeostasis. Peptides 2007, 28, 390–395.
- Madsen, L.R.; Baggesen, L.M.; Richelsen, B.; Thomsen, R.W. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: A Danish population-based matched cohort study. Diabetologia 2019, 62, 611–620.
- Alkharaiji, M.; Anyanwagu, U.; Donnelly, R.; Idris, I. Effect of Bariatric Surgery on Cardiovascular Events and Metabolic Outcomes in Obese Patients with Insulin-Treated Type 2 Diabetes: A Retrospective Cohort Study. Obes. Surg. 2019, 29, 3154–3164.
- Schauer, P.R.; Burguera, B.; Ikramuddin, S.; Cottam, D.; Gourash, W.; Hamad, G.; Eid, G.M.; Mattar, S.; Ramanathan, R.; Barinas-Mitchel, E.; et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann. Surg. 2003, 238, 467–484; discussion 484–465.
- Pournaras, D.J.; Osborne, A.; Hawkins, S.C.; Vincent, R.P.; Mahon, D.; Ewings, P.; Ghatei, M.A.; Bloom, S.R.; Welbourn, R.; le Roux, C.W. Remission of type 2 diabetes after gastric bypass and banding: Mechanisms and 2 year outcomes. Ann. Surg. 2010, 252, 966–971.
- Isbell, J.M.; Tamboli, R.A.; Hansen, E.N.; Saliba, J.; Dunn, J.P.; Phillips, S.E.; Marks-Shulman, P.A.; Abumrad, N.N. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010, 33, 1438–1442.
- Peck, B.C.E.; Seeley, R.J. How does ‘metabolic surgery’ work its magic? New evidence for gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 81–86.
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057.
- Kong, L.C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.L.; Zucker, J.D.; Dore, J.; Clement, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24.
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jorgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67.
- Liou, A.P.; Paziuk, M.; Luevano, J.M., Jr.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra141.
- Ricci, C.; Gaeta, M.; Rausa, E.; Macchitella, Y.; Bonavina, L. Early impact of bariatric surgery on type II diabetes, hypertension, and hyperlipidemia: A systematic review, meta-analysis and meta-regression on 6,587 patients. Obes. Surg. 2014, 24, 522–528.
- Ghanim, H.; Monte, S.; Caruana, J.; Green, K.; Abuaysheh, S.; Dandona, P. Decreases in neprilysin and vasoconstrictors and increases in vasodilators following bariatric surgery. Diabetes Obes. Metab. 2018, 20, 2029–2033.
- Fenske, W.K.; Dubb, S.; Bueter, M.; Seyfried, F.; Patel, K.; Tam, F.W.; Frankel, A.H.; le Roux, C.W. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: A 12-month prospective study. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2013, 9, 559–568.
- Aron-Wisnewsky, J.; Julia, Z.; Poitou, C.; Bouillot, J.L.; Basdevant, A.; Chapman, M.J.; Clement, K.; Guerin, M. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J. Clin. Endocrinol. Metab. 2011, 96, 1151–1159.
- Heffron, S.P.; Parikh, A.; Volodarskiy, A.; Ren-Fielding, C.; Schwartzbard, A.; Nicholson, J.; Bangalore, S. Changes in Lipid Profile of Obese Patients Following Contemporary Bariatric Surgery: A Meta-Analysis. Am. J. Med. 2016, 129, 952–959.
- Heffron, S.P.; Lin, B.X.; Parikh, M.; Scolaro, B.; Adelman, S.J.; Collins, H.L.; Berger, J.S.; Fisher, E.A. Changes in High-Density Lipoprotein Cholesterol Efflux Capacity After Bariatric Surgery Are Procedure Dependent. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 245–254.
- Heneghan, H.M.; Meron-Eldar, S.; Brethauer, S.A.; Schauer, P.R.; Young, J.B. Effect of bariatric surgery on cardiovascular risk profile. Am. J. Cardiol. 2011, 108, 1499–1507.
This entry is offline, you can click here to edit this entry!
