Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
In the search for effective biomaterials for bone repair, magnesium phosphate cements (MPCs) are nowadays gaining importance as bone void fillers thanks to their many attractive features that overcome some of the limitations of the well-investigated calcium-phosphate-based cements.
- magnesium phosphates
- cements
- bone tissue
- pastes
1. Introduction
MPCs can be defined as cements in which the binding phase is made of magnesium phosphate. The first publication concerning MPCs for applications in the biomedical field dates back to 1995 [48], but such materials were already known as fast-repairing cements for civil engineering applications [49]. The interest in MPCs rapidly grew in the last decade, as can be gathered by the number of publications as a function of the year for MPCs and CPCs: the attention towards the former markedly grew only recently, whereas the number of publications related to CPCs has been relatively constant for the last 10 years (see Figure 1a). From a commercial viewpoint, while dozens of CPC-based products are available, only one company (Bone Solutions, Inc., Colleyville, Texas, USA) is currently commercializing MPCs. Three types of products are available: Mg OsteoCrete, Mg OsteoInject, and Mg OsteoRevive. According to the producers, OsteoCrete is described as a “moldable, injectable magnesium-based bone void filler for trauma/orthopedic applications”, OsteoInject is recommended for sports medicine applications, and OsteoRevive is suitable for posterolateral spine applications. All these formulations are claimed to be moldable; radiopaque; fully synthetic; osteoconductive, with high compressive strength; and endowed with temperature-based setting control, excellent binding characteristics, thixotropic properties, and enhanced bone regeneration.
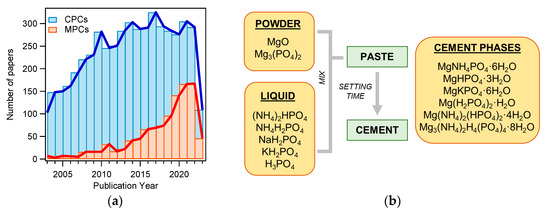
Figure 1. (a) Number of publications on the topic “calcium phosphate cement” (CPC) or “magnesium phosphate cement” (MPC) from 2003 to 2023 (Web of Science, updated in May 2023); (b) diagram of MPC preparation. The main powder and liquid components are listed, together with the main phases found in the set cements.
2. Preparation of MPCs
MPCs can be prepared using different types of powder and liquid precursors, as schematized in Figure 1b. The powder component often consists of MgO and, less frequently, Mg3(PO4)2 (tri-magnesium phosphate, TMP). The liquid is usually made of an aqueous solution of a phosphate salt (ammonium, sodium, or potassium) or phosphoric acid. Upon mixing, the two components react, forming a viscous and moldable paste. In time, due to dissolution and re-precipitation reactions, the system hardens, forming a compact and solid cement because of the entanglement of the newly formed crystals. The type of phase in the final material changes according to the precursors used and the setting conditions. The different magnesium-phosphate-based phase names, together with the relative solubility and Mg/P ratio, are given in Table 1, while Table 2 shows the precursors and the final phases reported in the literature for MPCs, for applications both as bone cements and in the construction field.
Table 1. Magnesium phosphates: chemical formulae, mineral names, solubility product constants at 25 °C, and Mg/P atomic ratios.
| Chemical Formula | Mineral Name | Solubility (Ksp) | Mg/P Atomic Ratio | Abbreviation |
|---|---|---|---|---|
| Mg3(PO4)2 | Farringtonite | 3.9 × 10−23 [50] | 1.5 | TMP |
| Mg3(PO4)2·8H2O | Bobierrite | 6.3 × 10−26 [51] | 1.5 | BOB |
| Mg3(PO4)2·22H2O | Cattiite 1 | 8 × 10−24 [51] | 1.5 | CAT |
| MgNH4PO4·H2O | Dittmarite | Unknown | 1 | DIT |
| MgHPO4·3H2O | Newberyite | 1.5 × 10−6 [51] | 1 | NEW |
| MgHPO4·7H2O | Phosphorrösslerite | 9.77 × 10−18 [50] | 1 | PHO |
| MgNH4PO4·6H2O | Struvite | 7.1 × 10−14 [52] | 1 | STR |
| MgKPO4·6H2O | K- Struvite | 2.4 × 10−11 [52] | 1 | KST |
| (NH4)2Mg3(HPO4)4·8H2O | Hannayite 2 | Unknown | 0.75 | HAN |
| (NH4)2Mg(HPO4)2·4H2O | Schertelite 2 | Unknown | 0.5 | SCH |
Table 2. Comprehensive list of MPC formulations reported in the literature for applications in the biomedical and construction fields. In the Reference column, the former are marked with “B”, the latter with “C”, and those marked with “N” did not explicitly state the application field. Formulations containing Ca-doped MPCs or Mg-doped CPCs were not included in the list. The papers listed are those resulting from a search conducted in Web of Science using the keywords “magnesium phosphate cements” in the “topic” section.
| Powder Component |
Phosphate Salt Aqueous Solution |
Additional Components |
P/L 1 | Final Crystalline Phases | References |
|---|---|---|---|---|---|
| MgO | NH4H2PO4 | - | - | STR + SCH + DIT | [54]—N |
| MgO | NH4H2PO4 | Silica | 8.3–20 g/mL | MgO + STR | [55]—N |
| MgO | NH4H2PO4 | Sand | 4 g/mL | MgO + STR | [56]—C |
| MgO | NH4H2PO4 + NaH2PO4 | Borax | 7.7 g/mL | MgO + STR + SCH | [57]—B |
| MgO | NH4H2PO4 + NaH2PO4 | Borax, Bi2O3 | 7.7 g/mL | MgO + STR + SCH | [58]—B |
| MgO | NH4H2PO4 | Borax + Zn + quartz | 5.6–7.1 g/mL | - | [59]—C |
| MgO | NH4H2PO4 + NaH2PO4 | Chitosan | 9 g/mL | MgO + STR + SCH + Na2Mg(HPO4)2 | [60]—B |
| MgO | NH4H2PO4 | Na2B4O7·5H2O | 8.3 g/mL | MgO + STR + DIT | [61]—N |
| MgO | NH4H2PO4 | Borax, acetic acid | 8.3 g/mL | MgO + STR + Mg(acetate) + NH4H2PO4 | [62]—C |
| MgO | NH4H2PO4 + NaH2PO4 | Borax | 7.7 g/mL | MgO + STR + SCH | [63]—B |
| MgO | NH4H2PO4 | Borax, acetic acid, sand | 8.3 g/mL | MgO + STR | [64]—C |
| MgO | NH4H2PO4 | Borax, sand, metakaolin | 4.3 g/mL | MgO + STR | [65]—C |
| MgO | NH4H2PO4 | Borax, Na5P3O10, metakaolin, sand, sodium polyacrylate | 5 g/mL | STR | [66]—C |
| MgO | NH4H2PO4 | Borax, Na5P3O10, acetic acid | 6.66 g/mL | MgO + STR | [67]—C |
| MgO | NH4H2PO4 | H3BO3 | - | MgO + STR + DIT | [68]—N |
| MgO + AMP | PVA solution | Mg granules | 0.5 g/mL | BOB | [69]—B |
| MgO | NH4H2PO4 | Na5P3O10, H3BO3, sand | 5.3–6.3 g/mL | - | [70]—C |
| MgO | NH4H2PO4 | Na5P3O10, borax, H3BO3, sand | - | - | [71]—C |
| MgO | NH4H2PO4 | Metakaolin, Al2O3, borax, sand |
11.1 g/mL | MgO + STR + Al phosphate hydrates | [72]—C |
| MgO | NH4H2PO4 | Borax, citric acid, quartz, foaming agent | 5.5–7.1 g/mL | MgO + STR + SiO2 | [73]—C |
| MgO | NH4H2PO4 | H3BO3 | 1.7 g/mL | MgO + STR | [74]—N |
| MgO | NH4H2PO4 | Fly ash, sand, borax, Na5P3O10 | 5 g/mL | MgO + STR | [75]—C |
| MgO | NH4H2PO4 | Borax, H2O2, Al2O3, zeolite |
2.1–2.6 g/mL | MgO + STR + NH4H2PO4 | [76]—N |
| MgO | H3PO4 | MgCl2 | 0.6–1 g/mL | Mg(OH)2·MgCl2·8H2O | [77]—B |
| MgO | KH2PO4 +NH4H2PO4 | Fly ash, borax, Na5P3O10, | 10 g/mL | MgO + STR + KST | [78]—C |
| MgO | NH4H2PO4 | retarder | - | - | [79]—B |
| MgO | KH2PO4, NH4H2PO4 | Borax | 7.1 g/mL | MgO + STR/KST + DIT | [80]—C |
| MgO | KH2PO4, NH4H2PO4 | Pluronic F127 | - | MgO, Mg(OH)2, STR, NEW | [81]—B |
| MgO | KH2PO4 + NH4H2PO4 | Al2O3, borax | 6.25–7.1 g/mL | MgO + STR + KST + alumina phosphate hydrate | [82]—N |
| MgO | KH2PO4 + NH4H2PO4 | H3BO3, Na5P3O10 | - | MgO + KST + KH2PO4 + lünebergite | [83]—C |
| MgO | KH2PO4, NaH2PO4 | Borax, sand | 5 g/mL | MgO + KST + NaST | [84]—C |
| MgO | KH2PO4 | Borax | 8.3–12.5 g/mL | MgO + KST + borax | [85]—C |
| MgO | KH2PO4 | Borax | 5–7.1 g/mL | MgO + KST | [86]—C |
| MgO | KH2PO4 | Borax | 0.1–0.4 g/mL | MgO + KST | [87]—C |
| MgO | KH2PO4 | - | 6.66 g/mL | MgO + KST | [88]—C |
| MgO | KH2PO4 | Borax | - | MgO + KST | [89]—C |
| MgO | KH2PO4 | Fly ash, H3BO3 | 3.6–3.8 g/mL | MgO + KST + SiO2 | [90]—C |
| MgO | KH2PO4, Na2HPO4·12H2O | Borax | - | MgO + KST + Na2Mg(HPO4)2 + K2Mg(HPO4)2·4H2O | [91]—C |
| MgO | KH2PO4 | Fly ash, borax, sand | 5–7.1 g/mL | MgO + KST | [92]—C |
| MgO | KH2PO4 | - | 2–6.66 g/mL | MgO + KST | [93]—C |
| MgO | KH2PO4 | - | 3.3 g/mL | MgO + KST | [94]—N |
| MgO | KH2PO4 | - | 5 g/mL | MgO + KST | [95,96]—C |
| MgO | KH2PO4 | Glucose | 3.3–5.5 g/mL | MgO + KST | [97]—B |
| MgO | KH2PO4 | - | 2 g/mL | MgO + KST | [98]—C |
| MgO | KH2PO4 | - | 2.3 g/mL | MgO + KST | [99]—N |
| MgO | KH2PO4 | - | 2 g/mL | MgO + KST | [100]—N |
| MgO | KH2PO4 | H3BO3 | 1 g/mL | Mg2KH(PO4)2·15H2O + KST | [101]—N |
| MgO | KH2PO4 | Borax, fly ash, sand | 4.3–6.2 g/mL | MgO + KST + SiO2 | [102]—C |
| MgO | KH2PO4 | Sand, fly ash, | 3.3 g/mL | - | [103]—C |
| MgO | KH2PO4 | Borax, NaHCO3 | 5.9 g/mL | MgO + KST | [104]—C |
| MgO | KH2PO4 | Acylic latexes | 2.3 g/mL | MgO + KST | [105,106]—C |
| MgO | KH2PO4 | Silanes, borax, Na5P3O10 | 9 g/mL | MgO + KST | [107]—C |
| MgO | KH2PO4 | Chondroitin sulfate | 2 g/mL | MgO + KST | [108]—B |
| MgO | KH2PO4 | Carboxymethyl chitosan | 2 g/mL | MgO + KST | [109]—B |
| MgO | KH2PO4 | - | 0.2–4 g/mL | MgO + KST+ PHO + Mg2KH(PO4)2·15H2O | [110]—C |
| MgO | KH2PO4 | Citric acid | 2 g/mL | MgO + KST | [111]—N |
| MgO | KH2PO4 | Borax, sand, sulphoaluminate cement | 6.25 g/mL | MgO + KST + Ca-phases |
[112]—C |
| MgO | KH2PO4 | Borax, fly ash | 7.14 g/mL | MgO + KST | [113]—C |
| MgO | KH2PO4 | - | 2.3 g/mL | MgO + KST | [114]—N |
| MgO | KH2PO4 | Al2(SO4)3·16H2O | 0.2–4 g/mL | MgO + KST + others | [115]—C |
| MgO | KH2PO4 | Borax, Zn(NO3)2 | 0.1–7.14 g/mL | MgO + KST | [116]—C |
| MgO | KH2PO4 | CaCO3, citric acid | 0.16 g/mL | MgO + KST + CaCO3 | [117]—B |
| MgO | KH2PO4 | - | 0.25–0.7 g/mL | MgO + KST | [118]—B |
| MgO | KH2PO4 | Borax, fly ash, silica fume, sand | 5.5 g/mL | - | [119]—C |
| MgO | KH2PO4 | Borax, ferroaluminates | 4.5 g/mL | MgO + KST + aluminates | [120]—C |
| MgO | KH2PO4 | CaSiO3, quartz, MgCl2 | 1.2–4 g/mL | MgO + KST + CaSiO3 + Mg2KH(PO4)2·15H2O | [121]—C |
| MgO | KH2PO4 | CaSiO3 | 4 g/mL | MgO + KST + BOB + MgKPO4·H2O + CaSiO3 |
[122]—C |
| MgO | KH2PO4 | - | 6.7 g/mL | MgO + KST + BOB | [123]—C |
| MgO | KH2PO4 | Borax, limestone, Na2HPO4·12H2O | 6.25 g/mL | MgO + KST + limestone | [124]—C |
| MgO | KH2PO4 + H3PO4 | Sucrose, Na5P3O10, hydroxyapatite |
3.3 g/mL | MgO + KST | [125]—B |
| MgO | KH2PO4 | Sucrose, borax, hydroxyapatite |
6.25 g/mL | MgO + KST | [126]—B |
| MgO | KH2PO4 | - | 6 g/mL | MgO + KST | [127]—B |
| MgO | KH2PO4 | Borax, NaH2PO4 | 5 g/mL | Na-KST | [128]—C |
| MgO | KH2PO4 | Borax, fly ash, sand, PVA fibers | 5.3–10 g/mL | - | [129]—C |
| MgO | KH2PO4 | Borax, sand, basalt | 2.2 g/mL | MgO + KST + MgCO3 + MgCO3·3H2O | [130]—C |
| MgO | KH2PO4 | Graphene oxide, borax, SDS | 7.14 g/mL | MgO + KST | [131]—C |
| MgO | KH2PO4 | Oxygen-carboxymethyl chitosan | 2 g/mL | MgO + KST | [132]—B |
| MgO | KH2PO4 | ZIF-8 | 2.5 g/mL | MgO + KST | [133]—B |
| MgO | KH2PO4, K2HPO4·3H2O | - | 2.7 g/mL | MgO + KST+ MgKPO4·H2O | [134]—C |
| MgO | KH2PO4 | H3BO3, polyurea aerogel | - | - | [135]—B |
| MgO | KH2PO4 | H3BO3, Al(NO3)3 | 0.01 g/mL | CAT + NEW | [136]—C |
| MgO | KH2PO4 | Laponite, sepiolite, halloysite | 2 g/mL | MgO + KST | [137]—B |
| MgO | KH2PO4 | Carboxymethyl chitosan, sodium alginate | 2 g/mL | MgO + KST | [138]—B |
| Mg(OH)2 | H3PO4 | Microwaves | 2.5 g/mL | NEW | [139]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 3 g/mL | TMP + STR + NEW | [140]—B |
| TMP + STR | (NH4)2HPO4 | - | 2.1 g/mL | TMP + STR | [141,142]—B |
| BOB | (NH4)2HPO4 | TWEEN20, PU foam | - | TMP + STR | [143]—B |
| TMP | (NH4)2HPO4 | (NH4)2C6H6O7 | 1–3.3 g/mL | TMP + STR | [144]—B |
| TMP | (NH4)2HPO4/K2HPO4/ H3PO4 |
HPMC | 2 g/mL | STR, KST, NEW | [145]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 2–3 g/mL | TMP + STR | [46]—B |
| TMP | (NH4)2HPO4 | HPMC | 1.5 g/mL | TMP + STR | [146]—B |
| TMP | Phytic acid | 3 g/mL | TMP + NEW | [147]—B | |
| TMP | (NH4)2HPO4 | - | 1–2 g/mL | TMP + STR | [148]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 0.33–2 g/mL | HAN | [149]—B |
| TMP | (NH4)2HPO4 | HPMC + NaCl | 1.33 g/mL | TMP + STR | [150]—B |
| TMP 2 | H2O | - | 1.5 g/mL | TMP + CAT | [151]—B |
| TMP | (NH4)2HPO4 | HPMC + gelatin | - | STR | [152]—B |
| TMP | (NH4)2HPO4 | Halloysite | 1.5 g/mL | TMP + STR | [153]—C |
| TMP | (NH4)2HPO4 + NH4H2PO4 | - | 2–3 g/mL | TMP + STR | [154]—B |
| TMP | (NH4)2HPO4 | HPMC + indene | 1.33 g/mL | TMP + STR | [155]—B |
| TMP | (NH4)2HPO4 | ammonium citrate | 2 g/mL | TMP + STR | [156]—B |
| TMP | (NH4)2HPO4 + NH4H2PO4/K2HPO4 + KH2PO4 | - | 0.6–1.5 g/mL | TMP + STR + NEW + SCH + KST |
[157]—B |
| TMP | (NH4)2HPO4 | Gelatin microparticles | 1.5 g/mL | TMP + STR | [158]—B |
| TMP | (NH4)2HPO4 | Borax | 1.5 g/mL | TMP + STR | [159]—N |
| TMP | KH2PO4 | - | 2–3 g/mL | TMP + KST + NEW | [160]—B |
| MgO+ TMP | Phytic acid | - | 2 g/mL | TMP + NEW | [161]—B |
| MgO+ TMP | Phytic acid | - | 1.71–2 g/mL | MgO + TMP + NEW | [162,163]—B |
| AMP | H2O | PVA | 0.4–0.6 g/mL | CAT | [164]—B |
The data in Table 2 show that the majority of the works carried out so far have used MgO as a precursor powder, which is often reacted with NH4H2PO4 or KH2PO4 to form STR or KST as binding phases, respectively. TMP has been used to a lesser extent, mostly for reactions with (NH4)2HPO4 resulting in STR. In all cases, a significant amount of reacting powder, either MgO or TMP, was present in the final cement matrix.
Concerning the powder precursors, different factors should be taken into account when using either MgO or TMP. The reactivity of the MgO, for instance, is a crucial parameter, since MPCs can be obtained only using dead burned MgO, i.e., treated at temperatures above 1300 °C. Not only is the calcination temperature important to control the setting process of MgO-based MPCs, but also the deformation ratio of MgO grains, which is the ratio of the experimental specific surface area vs. the theoretical one [56]. This parameter is connected to the disorder of the MgO grains’ surfaces, affecting their reactivity and, as a consequence, the cement setting time. The reactivity of MgO can be easily estimated with the citric acid test, in which a MgO slurry is mixed with a citric acid solution containing phenolphthalein as an indicator [165]. According to the time needed by the formed magnesium hydroxide to neutralize the citric acid solution (indicated by the color change of the solution from white to pink), MgO is classified as having high, medium, or low reactivity. Dead burned MgO is characterized by a neutralization time greater than 900 s. The low reactivity of MgO is necessary to guarantee a homogeneous mix between the powder and the liquid component when preparing a cement; even so, it is often necessary to use a retarder to obtain the desired setting time. It should be pointed out that, especially for MPCs applied in the construction field, MgO is typically not pure and can also contain certain amounts of CaO, SiO2, Al2O3, Fe2O3, K2O, Na2O, TiO2, SO3, and P2O5 [61,62,74,80,95,97,98,131].
TMP, on the other hand, is typically obtained from the calcination of Mg(OH)2 and MgHPO4·3H2O above 1000 °C, followed by grinding and sieving [144,145,148].
The liquid component of an MPC formulation is often an aqueous solution of ammonium or potassium phosphate. When using the former, especially in combination with MgO, the release of ammonia and the highly exothermic reaction that occurs during the hydration process can be drawbacks. In this case, replacing the ammonium with potassium salts, which do not result in the above problems, is a viable option [95,166].
The preparation procedure is similar for all MPCs, though a difference according to the type of precursor can be found: MgO is often mixed with the phosphate salt as a solid, and water is then added to the formulation; in this case, the w/c (water/cement), w/b (water/binder), or w/s (water/solid) ratio is specified. When TMP is used, the phosphate salt is first dissolved in water, and this solution is then mixed with the TMP powder: the proportions between the two components are given as the P/L (powder/liquid) ratio. Regardless of the precursor and the additional materials included in the formulation, the cements are prepared by mixing, molding, and hardening, often in controlled conditions of relative humidity.
In MgO-based formulations, the phosphate salt is often included as a solid within the powder component. When the mixture comes into contact with water, the phosphate salt immediately dissolves, leading to the release of protons and decreasing the pH of the solution [19]. According to the mechanism proposed by Wagh and Jeong [167], MgO slowly dissolves from the edges of the grains, leading to the formation of an intermediate aquosol. Afterwards, the acid–base reaction with the phosphate groups in the solution results in a gel that forms a layer on the surface of the undissolved MgO grains. In time, this gel thickens and hardens due to the growth of the crystalline binding phases, leading to the final hydration product. It is worth noting that some transient phases among those reported in Table 1 might form during this process [168]. The reaction mechanism occurring when using TMP as a precursor powder has been investigated to a lesser extent. In a similar way, it is believed that the reaction occurs on the surface of the TMP grains, where the Mg2+ ions react with phosphate and ammonium ions from the aqueous component, resulting in the precipitation and entanglement of the binding phases [159].
3. Properties of MPCs
Many properties make MPCs attractive in the field of bone cements. One of these is their setting time, which is typically in line with clinical needs. The setting time of a bone cement can be roughly defined as the time needed for the cement paste to become strong enough to resist an applied force [6]. This parameter can be measured with different tests, such as the Gillmore or Vicat tests, the former being the most commonly used for MPCs designed as bone cements. During the Gillmore test, the initial (t1) and final (t2) setting times of the paste are determined: t1 is the time needed for the cement to bear a thin and light (113 g) needle without appreciable indentation, while t2 is the time needed for the cement to bear a thick (454 g) and heavy needle without appreciable indentation (ASTM C266-21). There is clinical significance behind these values, as the cement paste should be implanted before t1 and the wound should be closed after t2 [7]. It is therefore important that the paste sets slowly enough to allow for mixing and molding/injecting in the bone defect. At the same time, it should not set too slowly, so that the wound can be quickly closed and the set cement provide the needed mechanical support. In the literature, it has been suggested that the ideal ranges for t1 and t2 are 3 ≤ t1 < 8 min and t2 ≤ 15 min, respectively [7]. As can be observed from the data reported in Figure 3a, most MPC formulations are able to set within the desired time frame, irrespective of the precursor used. The setting time depends on several parameters, namely the powder’s characteristics (size, reactivity, and specific surface area); the aqueous solution concentration; the P/L ratio; the temperature; and the humidity [12]. In some cases, if the modulation of these parameters is not sufficient or possible, the setting time of the paste can be extended through the inclusion of retarders (see Section 5.1).
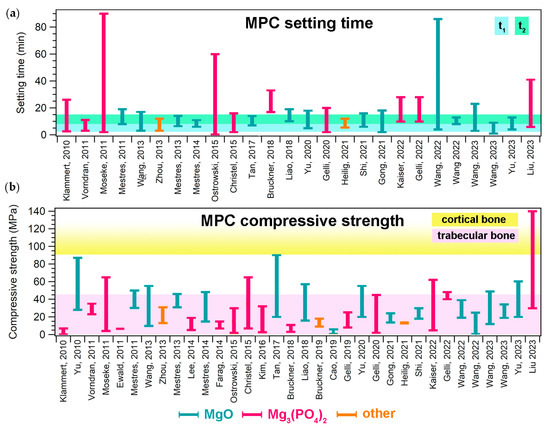
Figure 3. (a) Setting time ranges given in the literature for MPCs designed for biomedical applications; for each paper, the bar ranges between the minimum and maximum values reported for a specific formulation. The colored area indicates the ideal t1 and t2 ranges, according to [7]. (b) Compressive strength ranges reported in the literature for MPCs designed for biomedical applications; for each paper, the bar ranges between the minimum and maximum values reported for a specific formulation. The colored areas indicate the compressive strength ranges of trabecular (2–45 MPa) and compact (90–230 MPa) bone [8]. In both graphs, the different bar colors indicate the precursor powder: light blue (MgO), pink (Mg3(PO4)2, and orange (others). The references mentioned in the figure are the following: [33,57,58,60,63,79,81,97,108,109,117,118,132,133,134,137,138,139,140,141,144,145,146,147,148,149,150,151,152,156,157,158,161,162].
Another important feature of bone cement pastes is their cohesion, which is defined as their ability to harden in a fluid without disintegration [169]. The cohesion is connected to the “anti-washout ability” of the cement, which is typically determined in a dynamic environment, while “cohesion tests” are carried out in static conditions [6]. Cohesion is an important feature, since MPCs, when used to fill a bone defect, will come into contact with biological fluids before they are completely hardened: an ideal cement should then be able to retain its shape and harden even when in contact with aqueous media. This property has been well addressed in the CPC literature [170,171,172,173,174], whereas for MPCs it is often neglected, and very few publications have discussed this aspect. While developing antimicrobial MPCs for dental applications, Mestres et al. determined their cohesion time as the time needed by the cement to no longer disintegrate when immersed in an aqueous solution by visually inspecting cement disks soaked in distilled water [63]. They found that less than 7 min was required by all the tested formulations to attain the cohesion time. Polymeric additives such as chitosan have been reported to improve the cohesion of pastes when in contact with water [60]. Yu et al. found that an intermediate concentration of carboxymethyl chitosan prevented paste disintegration upon injection in Ringer’s solution [109], as well as when used in combination with alginate [138]. Foaming agents can also help in improving anti-washout resistance [117]. Recently, Liu and colleagues reported remarkable results in the direct extrusion of a TMP-K2HPO4 cement in simulated body fluid without the use of additives: the cement, after shaking for 15 min at 37 °C, did not show any significant powdering or dissolution, proving its excellent anti-washout resistance [160]. Heilig et al. recently showed that MPCs based on a combination of TMP and MgO reacted with phytic acid also displayed notable cohesion properties, as they could be manually injected into water without the appearance of any streaks on the water surface [162].
The cohesion is strictly connected to the injectability of a cement paste. The injectability of MPCs is one of their most appealing features from a clinical perspective, as it allows one to perform minimally invasive surgeries, which are in general associated with reduced pain, shorter hospital stays, and fewer complications compared to traditional surgeries. In addition, the possibility of injecting a paste rather than implanting a block or solid materials allows in principle for improved bone-to-implant contact [8].
Injectability depends on both the paste properties and the injection parameters. When assessing this parameter, the clinically relevant conditions in which bone cements are applied should be kept in mind in the design of the experiment: the needles used in percutaneous surgeries such as vertebroplasty or kyphoplasty usually range from 13- to 8-gauge (internal diameters of 1.80–3.43 mm) and from 10 to 15 cm in length [175]. The injectability of MPC pastes is a property frequently evaluated in the literature. Typically, the % of extruded paste with respect to the amount initially loaded in the syringe is calculated, and the injectability % is obtained. The injection can be performed manually [109,158] or in controlled conditions, using specific loads and testing machines [58,160,162]. Mestres et al. compared Na-MPCs, NH4-MPCs, and a mixed system, observing that Na-based MPCs had a higher injectability % [58]. The inclusion of carboxymethyl chitosan in the MPC has been reported to extend the injectability time, in a concentration-dependent fashion, as well as the inclusion of alginate [138]. The injectability % and the injection time both increased when including a foaming agent based on calcium carbonate and citric acid [117]. In TMP-based formulations, the work of Moseke et al. showed that the partial replacement of (NH4)2HPO4 with (NH4)2citrate led to an improvement in the injectability % [144]. MPCs obtained from the combination of both TMP and MgO also displayed excellent performances in terms of injectability %, reaching 100% at forces below 300 N [162]. In MPCs, a low injectability % can be due to several reasons, namely a short setting time (i.e., the paste hardens too fast during the extrusion process); a high viscosity; and phase separation (or filter pressing) phenomena. In the early stages of cement formation, the unset cement consists of a powder component dispersed in a liquid. If, upon injection, the liquid phase travels at a faster rate than the powder particles, the extrudate will contain a much higher liquid content than the paste initially loaded into the syringe, eventually leading to a cement with markedly different properties. Phase separation phenomena are well-established for CPCs [9], whereas in the MPC literature, the characteristics of the extruded pastes are often neglected.
While the properties of MPC pastes are fundamental for the administration of the material in the body, the characteristics of the set cements are of course crucial to determine their performance when implanted in bone in terms of biocompatibility, mechanical integrity, dissolution behavior, and the interplay with bone cells.
Concerning the mechanical properties of MPCs, it is important to remark that such materials are classified as unsuitable for load-bearing applications [12,14]. They are in fact intended not to replace large regions of bone to create grafts, but rather to provide a support to repair small bone defects and damage. Nonetheless, it is important that in this process they are well integrated in the bone, displaying suitable mechanical properties to provide support. The mechanical property that is most frequently evaluated in the MPC literature is compressive strength. Figure 3b shows the ranges of compressive strength that have been reported for MPCs in the biomedical field, compared to those associated with cortical and trabecular bone: the majority of the MPCs reported in the literature could match the compressive strength of trabecular bone (2–45 MPa) but did not reach 90 MPa, which is the minimum value for cortical bone. [8].
The mechanical properties are intimately connected to the microstructure and porosity of an MPC. It has been well established that a strategy to improve the mechanical properties of MPCs, and cements in general, is to reduce their porosity. Figure 4 shows a plot of the compressive strength as a function of the porosity % of different MPC formulations extracted from the literature.
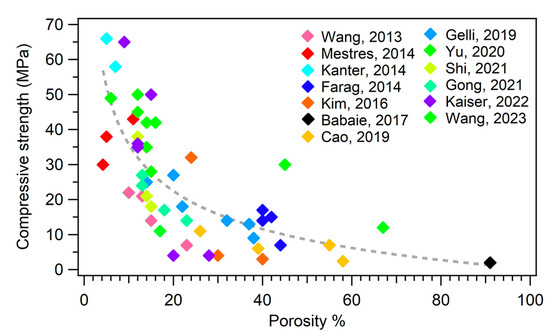
Figure 4. Plot of the compressive strength as a function of porosity %. The different colors of the markers refer to different publications, as reported in the legend. The dashed line is a guide for the eye. The references mentioned in the figure are the following: [46,58,69,81,108,109,118,132,137,148,150,152,157].
One might therefore think that minimizing the porosity of the material could be an effective strategy to boost the performance of an MPC. However, a decrease in the porosity of a cement to enhance the mechanical properties might be detrimental for the biological response of the material: a porous cement is in fact fundamental to favor bone ingrowth and cement degradation, which is connected to in vivo resorbability.
In principle, porosities on different length scales should be present in cements to achieve favorable bone integration. This aspect was recently reviewed by Lodoso-Torrecilla et al. for CPCs [8], highlighting that these materials possess an intrinsic microporosity resulting from the entanglement of the crystals formed during the setting process. This microporous structure enables the flow of biological fluids and protein adsorption but does not allow for bone cell permeation and ingrowth. The pore size generally considered adequate for bone regeneration is at least 100 μm, as smaller pores may result in the formation of unmineralized or fibrous tissue and may not allow for the growth of blood vessels [178,179]. To achieve this, macropores must be induced on purpose in the MPC matrix. In the literature, few attempts have been described for the preparation of macroporous MPCs: zinc powder [59], also in combination with a chemical foaming agent [73]; sodium bicarbonate [104]; and protein-based foaming agents [103] have been used, even though none of these materials were designed for application in the biomedical field. Macroporous MPCs for orthopedic applications have been obtained using biodegradable Mg particles as porogens during cement setting [69], polyurethane foams [143], and gelatin microparticles [158].
An effective solution to obtain pores of different sizes in MPCs consists in the inclusion of NaCl granules of various granulometries in 3D printed scaffolds: additive manufacturing allows for the creation of scaffolds with a tailored size and the building of macro-sized pores > 300 µm. Salt leaching leads to the dissolution of NaCl grains, leaving behind a porous structure with pores below 25 µm and between 25 and 53 µm produced using different sizes of NaCl templates [150].
An increase in porosity is inevitably connected to a decrease in the mechanical properties, so one should carefully design the formulation parameters of MPCs according to one’s specific needs.
The enhancement of cement porosity is, for instance, important when the material needs to be quickly resorbed in vivo. The fast degradation rate of MPCs is one of their main advantages when compared to CPCs. Magnesium phosphates are typically more soluble than their calcium counterparts, and this is often described as one of their most appealing features. In fact, MPCs are not expected to endure when clinically injected: on the contrary, they should be resorbed and replaced over time by the body’s own regenerated tissues, providing temporary support. The dissolution timeframe should ideally match the body’s bone reforming ability. In fact, the overly rapid degradation of the MPC might induce some side effects, as in the initial stages the cement should provide structural integrity to the bone defect, allowing for the healing mechanisms to take place. In vivo degradation can take place through two pathways: (i) passive degradation by the dissolution of the cement matrix into the biological fluids, and (ii) active degradation due to cellular activity. The first pathway is often assessed by incubating cements in aqueous fluids, such as PBS, SBF, or Tris-HCl buffer at pH 7.4, and evaluating their weight loss. Although such experiments do not directly translate to in vivo degradation rates, they are a valuable indication of the comparative rate of dissolution between different material compositions [12]. it is useful to provide a few examples to demonstrate the different dissolution behaviors of the two materials. For instance, Wu et al. developed mixed CPC–MPC formulations and studied their degradation in SBF over 90 days [180]. They found that the CPC lost less than 5% of its initial weight, while the MPC reached a weight loss of about 60%. Interestingly, the intermediate formulations showed intermediate dissolution rates, and the mixed cement provided faster and more effective osteogenesis in the defect area compared to the pure CPC. Another work from Wu et al. provided similar results [181]. Concerning pure MPCs, Mestres et al., comparing cements prepared using either sodium or ammonium phosphate as the aqueous phase, showed that upon immersion in PBS for 60 days, the weight loss at the end of the experiment was 4.3%, 5.2%, and 2.4%wt for NH4-MPC, Na-MPC, and NH4 + Na-MPC, respectively [58]. Higher dissolution values were recently reported by Yu et al. [138], who incubated MPCs modified with carboxymethyl chitosan and alginate in saline solution for 28 days and observed a weight loss % between ~15% and 18%, depending on the polymer concentrations in the cements. MPCs recently obtained by mixing TMP with potassium phosphate and incubated in Tris-HCl buffer (pH = 7.4) showed a weight loss % of about 12 % after 28 days of the experiment [160]. An interesting work from Kaiser et al. [157] compared the dissolution behavior of MPCs based on STR and KST with a reference of calcium-deficient hydroxyapatite: after incubation for 18 days in PBS, the STR and KST cements lost 1% and 8% of their initial weight, respectively, while the hydroxyapatite reference did not show any sign of degradation. Similar results were obtained by Ewald et al., who compared the weight loss of cements made of STR with two calcium phosphate references, brushite and calcium-deficient hydroxyapatite, upon incubation in PBS for 20 h: the hydroxyapatite-based material did not dissolve, while both the brushite and STR showed a cumulative dissolution normalized to the cement surface of approximately 2 mg/cm2 and 8 mg/cm2, respectively [141]. On the other hand, Cao et al. compared the degradation % of STR, NEW, and brushite-based scaffolds in PBS, showing that NEW and brushite slowly degraded over time, reaching ~22% and ~10% weight loss after 4 weeks, respectively [81]; STR was unexpectedly found to gain about 22% of its initial weight, and the authors attributed this result to the formation of Mg(OH)2 due to the reaction between the MgO precursor present in the cement and the water in the PBS.
Lee et al. studied the degradation of MgP scaffolds prepared using 3D printing [146]: PBS was used as the incubation medium, and after 20 days the materials retained 92% of their initial weight. Recently, Wang et al. observed that the presence of different clay minerals in MPC formulations modified their dissolution behavior in SBF and PBS at 28 days [137]. When amorphous magnesium phosphate was used as a powder precursor, the cements soaked in SBF for 8 days lost a considerable amount of weight, ranging from about 24% to 50%, depending on the quantity of PVA included in the formulation [164]. High dissolution values have also been reported in MPC–ZIF8 composites, which showed a weight loss between 39 and 46% upon incubation for 28 days in Tris-HCl buffer [133]. Similar experimental conditions (Tris-HCl buffer for 28 days) led to different results in studies by Gong et al., who observed a weight loss between 12 and 15 % in MPCs modified with oxygen-carboxymethyl chitosan [132], and Yu et al., showing a similar level of degradation for MPCs with carboxymethyl chitosan [109]. Wang et al. implemented the same experimental conditions and recently reported a weight loss between 15 and 22 % in MPCs prepared with a foaming agent [117], similar to the work of Shi et al. [108].
Such variations in the observed dissolution/degradation values can be attributed to not only the different formulations, but also the variety of experimental conditions used (the incubation medium, duration of the experiment, refreshing of the incubating solutions, etc.).
The study of the dissolution/degradation of cements in water is often accompanied by a study of the release kinetics of the constituent ions [58,109,138,146,157].
Finally, it is worth recalling that the slow degradation rate attributed to CPCs is mainly restricted to the apatitic ones, whereas brushite-based CPCs can dissolve more easily [182]. However, upon the dissolution of brushite, low-solubility phases might re-precipitate in vivo [46,47]. The presence of Mg2+ in cements prevents this issue, as Mg2+ ions hamper the crystallization of apatitic phases [183].
In summary, MPCs possesses many attractive features for the development of bone cement and are endowed with a higher strength, porosity, and resorption rate than CPC implants, while preserving good biocompatibility [19]. It is also evident that the enhancement of a specific property might often be detrimental to others, and researchers should carefully design materials according to their specific needs.
This entry is adapted from the peer-reviewed paper 10.3390/jfb14080424
This entry is offline, you can click here to edit this entry!
