Artificial Intelligence (AI) describes computer systems able to perform tasks that normally require human intelligence, such as visual perception, speech recognition, decision-making, and language translation. AI can be applied in many different areas, such as econometrics, biometry, e-commerce, and the automotive industry. AI has found its way into healthcare as well, helping doctors make better decisions, localizing tumors in magnetic resonance images, reading and analyzing reports written by radiologists and pathologists, and much more. However, AI has one big risk: it can be perceived as a “black box”, limiting trust in its reliability, which is a very big issue in an area in which a decision can mean life or death. As a result, the term Explainable Artificial Intelligence (XAI) has been gaining momentum. XAI tries to ensure that AI algorithms (and the resulting decisions) can be understood by humans.
- XAI
- AI
- artificial intelligence
- healthcare
- medicine
- explainability
- data science
- machine learning
- deep learning
- neural networks
1. Introduction
2. Central Concepts of XAI
2.1. From “Black Box” to “(Translucent) Glass Box”
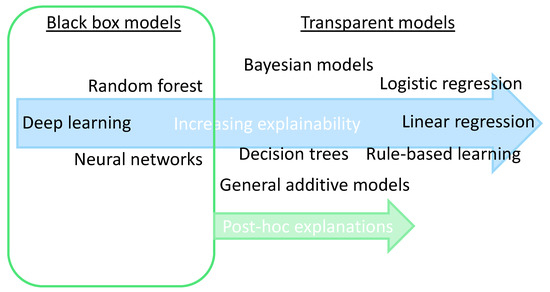
2.2. Explainability: Transparent or Post-Hoc
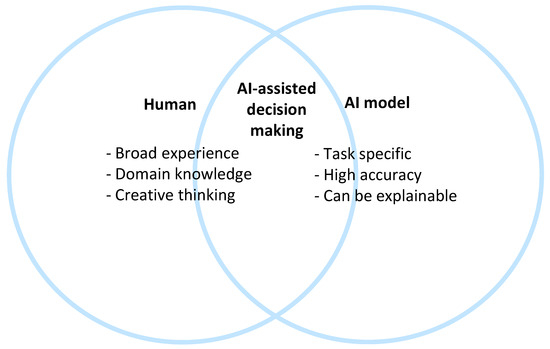
2.3. Collaboration between Humans and AI
2.4. Scientific Explainable Artificial Intelligence (sXAI)
2.5. Explanation Methods: Granular Computing (GrC) and Fuzzy Modeling (FM)
This entry is adapted from the peer-reviewed paper 10.3390/ai4030034
References
- Joiner, I.A. Chapter 1—Artificial intelligence: AI is nearby. In Emerging Library Technologies; Joiner, I.A., Ed.; Chandos Publishing: Oxford, UK, 2018; pp. 1–22.
- Hulsen, T. Literature analysis of artificial intelligence in biomedicine. Ann. Transl. Med. 2022, 10, 1284.
- Yu, K.-H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731.
- Hulsen, T.; Jamuar, S.S.; Moody, A.; Karnes, J.H.; Orsolya, V.; Hedensted, S.; Spreafico, R.; Hafler, D.A.; McKinney, E. From Big Data to Precision Medicine. Front. Med. 2019, 6, 34.
- Hulsen, T.; Friedecký, D.; Renz, H.; Melis, E.; Vermeersch, P.; Fernandez-Calle, P. From big data to better patient outcomes. Clin. Chem. Lab. Med. (CCLM) 2022, 61, 580–586.
- Biswas, S. ChatGPT and the Future of Medical Writing. Radiology 2023, 307, e223312.
- Celi, L.A.; Cellini, J.; Charpignon, M.-L.; Dee, E.C.; Dernoncourt, F.; Eber, R.; Mitchell, W.G.; Moukheiber, L.; Schirmer, J.; Situ, J. Sources of bias in artificial intelligence that perpetuate healthcare disparities—A global review. PLoS Digit. Health 2022, 1, e0000022.
- Hulsen, T. Sharing Is Caring-Data Sharing Initiatives in Healthcare. Int. J. Environ. Res. Public Health 2020, 17, 3046.
- Vega-Márquez, B.; Rubio-Escudero, C.; Riquelme, J.C.; Nepomuceno-Chamorro, I. Creation of synthetic data with conditional generative adversarial networks. In Proceedings of the 14th International Conference on Soft Computing Models in Industrial and Environmental Applications (SOCO 2019), Seville, Spain, 13–15 May 2019; Springer: Cham, Switzerlnad, 2020; pp. 231–240.
- Gunning, D.; Stefik, M.; Choi, J.; Miller, T.; Stumpf, S.; Yang, G.Z. XAI-Explainable artificial intelligence. Sci. Robot. 2019, 4, eaay7120.
- Rai, A. Explainable AI: From black box to glass box. J. Acad. Mark. Sci. 2020, 48, 137–141.
- Loyola-Gonzalez, O. Black-box vs. white-box: Understanding their advantages and weaknesses from a practical point of view. IEEE Access 2019, 7, 154096–154113.
- Ribeiro, M.T.; Singh, S.; Guestrin, C. Why should I trust you?: Explaining the predictions of any classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144.
- Gerlings, J.; Jensen, M.S.; Shollo, A. Explainable AI, but explainable to whom? An exploratory case study of xAI in healthcare. In Handbook of Artificial Intelligence in Healthcare: Practicalities and Prospects; Lim, C.-P., Chen, Y.-W., Vaidya, A., Mahorkar, C., Jain, L.C., Eds.; Springer International Publishing: Cham, Switzeralnd, 2022; Volume 2, pp. 169–198.
- Arrieta, A.B.; Díaz-Rodríguez, N.; Del Ser, J.; Bennetot, A.; Tabik, S.; Barbado, A.; García, S.; Gil-López, S.; Molina, D.; Benjamins, R. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion 2020, 58, 82–115.
- Phillips, P.J.; Hahn, C.A.; Fontana, P.C.; Broniatowski, D.A.; Przybocki, M.A. Four Principles of Explainable Artificial Intelligence; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020; Volume 18.
- Vale, D.; El-Sharif, A.; Ali, M. Explainable artificial intelligence (XAI) post-hoc explainability methods: Risks and limitations in non-discrimination law. AI Ethics 2022, 2, 815–826.
- Bhattacharya, S.; Pradhan, K.B.; Bashar, M.A.; Tripathi, S.; Semwal, J.; Marzo, R.R.; Bhattacharya, S.; Singh, A. Artificial intelligence enabled healthcare: A hype, hope or harm. J. Fam. Med. Prim. Care 2019, 8, 3461–3464.
- Zhang, Y.; Liao, Q.V.; Bellamy, R.K.E. Effect of Confidence and Explanation on Accuracy and Trust Calibration in AI-Assisted Decision Making. In Proceedings of the 2020 Conference on Fairness, Accountability, and Transparency, Barcelona, Spain, 27–30 January 2020; pp. 295–305.
- Antoniadi, A.M.; Du, Y.; Guendouz, Y.; Wei, L.; Mazo, C.; Becker, B.A.; Mooney, C. Current Challenges and Future Opportunities for XAI in Machine Learning-Based Clinical Decision Support Systems: A Systematic Review. Appl. Sci. 2021, 11, 5088.
- Amann, J.; Blasimme, A.; Vayena, E.; Frey, D.; Madai, V.I.; the Precise, Q.c. Explainability for artificial intelligence in healthcare: A multidisciplinary perspective. BMC Med. Inform. Decis. Mak. 2020, 20, 310.
- Durán, J.M. Dissecting scientific explanation in AI (sXAI): A case for medicine and healthcare. Artif. Intell. 2021, 297, 103498.
- Cabitza, F.; Campagner, A.; Malgieri, G.; Natali, C.; Schneeberger, D.; Stoeger, K.; Holzinger, A. Quod erat demonstrandum?—Towards a typology of the concept of explanation for the design of explainable AI. Expert Syst. Appl. 2023, 213, 118888.
- Holzinger, A.; Saranti, A.; Molnar, C.; Biecek, P.; Samek, W. Explainable AI methods—A brief overview. In Proceedings of the xxAI—Beyond Explainable AI: International Workshop, Held in Conjunction with ICML 2020, Vienna, Austria, 12–18 July 2020; Holzinger, A., Goebel, R., Fong, R., Moon, T., Müller, K.-R., Samek, W., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 13–38.
- Bargiela, A.; Pedrycz, W. Human-Centric Information Processing through Granular Modelling; Springer Science & Business Media: Dordrecht, The Netherlands, 2009; Volume 182.
- Zadeh, L.A. Fuzzy sets and information granularity. In Fuzzy Sets, Fuzzy Logic, and Fuzzy Systems: Selected Papers; World Scientific: Singapore, 1979; pp. 433–448.
- Keet, C.M. Granular computing. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; p. 849.
- Novák, V.; Perfilieva, I.; Dvořák, A. What is fuzzy modeling. In Insight into Fuzzy Modeling; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 3–10.
- Mencar, C.; Alonso, J.M. Paving the way to explainable artificial intelligence with fuzzy modeling: Tutorial. In Proceedings of the Fuzzy Logic and Applications: 12th International Workshop (WILF 2018), Genoa, Italy, 6–7 September 2018; Springer International Publishing: Cham, Switzerland, 2019; pp. 215–227.
- Zhang, C.; Li, D.; Liang, J. Multi-granularity three-way decisions with adjustable hesitant fuzzy linguistic multigranulation decision-theoretic rough sets over two universes. Inf. Sci. 2020, 507, 665–683.
- Zadeh, L.A. Toward a theory of fuzzy information granulation and its centrality in human reasoning and fuzzy logic. Fuzzy Sets Syst. 1997, 90, 111–127.
- Zhang, C.; Li, D.; Liang, J.; Wang, B. MAGDM-oriented dual hesitant fuzzy multigranulation probabilistic models based on MULTIMOORA. Int. J. Mach. Learn. Cybern. 2021, 12, 1219–1241.
- Zhang, C.; Ding, J.; Zhan, J.; Sangaiah, A.K.; Li, D. Fuzzy Intelligence Learning Based on Bounded Rationality in IoMT Systems: A Case Study in Parkinson’s Disease. IEEE Trans. Comput. Soc. Syst. 2022, 10, 1607–1621.
- Solayman, S.; Aumi, S.A.; Mery, C.S.; Mubassir, M.; Khan, R. Automatic COVID-19 prediction using explainable machine learning techniques. Int. J. Cogn. Comput. Eng. 2023, 4, 36–46.
- Gao, S.; Lima, D. A review of the application of deep learning in the detection of Alzheimer's disease. Int. J. Cogn. Comput. Eng. 2022, 3, 1–8.
