Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Psychology, Biological
Schizophrenia is a complex psychiatric condition that may involve immune system dysregulation. Microglia are the resident brain innate immune cells that have been implicated in host defense against neurotropic pathogens, brain development, and neurodegenerative disorders.
- schizophrenia
- microglia
- neuroinflammation
- immunological dysfunction
1. Introduction
Schizophrenia (SCZ), a chronic psychiatric illness that affects approximately 24 million people worldwide, is characterized by the hallmark “positive” symptoms of hallucinations and delusions and “negative” symptoms of apathy, anhedonia, avolition, and emotional and cognitive impoverishment [1]. This debilitating disorder imposes a significant risk of physical and mental health complications, ranging from coronary heart disease to suicidal behavior (SB), highlighting reciprocal relationships between somatic psychic implications in neuro-psychiatric conditions [2]. SCZ diagnosis is difficult due to the spectral nature of the illness and the complex progression of its clinical manifestation. Patients with SCZ often present with subtle irritation/behavioral changes in the prodromal phase, followed by the onset of psychosis. Before 2013, SCZ was categorized into various subtypes (paranoid, disorganized, catatonic, undifferentiated, and residual) based on specific clinical presentations. However, this discrete division of the illness was supplemented by the concept of SCZ being a spectral disease that includes schizoaffective, schizophreniform, and schizotypal personality disorders [3].
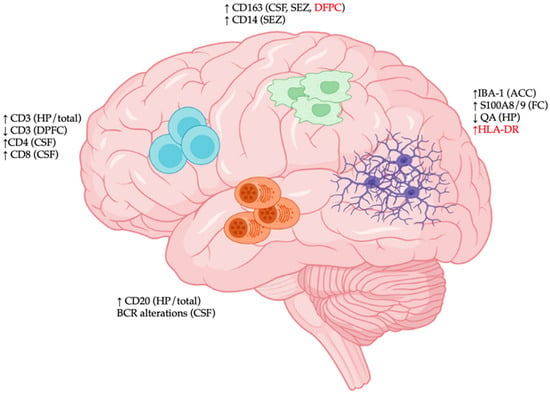
2. Cellular Constituents of CNS Immunological Aberrations in SCZ
Following reports of elevated neuroinflammation in SCZ, an initial hypothesis stated that CNS immune disturbance may be involved in SCZ pathogenesis. The subsequent discovery of risk factors in genes associated with immune-mediated neurodevelopmental processes and the identity of CNS immune cells provided further support for the immunological origin of SCZ (Figure 1).

Figure 1. Immunological disturbances in the central nervous system (CNS) of patients with schizophrenia (SCZ). Region-specific immunological changes in CNS tissues of SCZ patients are characterized by (1) elevated expression of various activation markers of microglia (S100/A8, HLA-DR), decreased expression of neuroprotective quinolinic acid (QA), and increased microgliosis (IBA-1 density); (2) increased expression of macrophage markers (CD14 and CD163); (3) dynamic trafficking of various T cell populations (CD3/CD4/CD8); and (4) CD20+ B cell accumulation and altered B cell receptor (BCR) repertoire. Abbreviations: ACC: anterior cingulate cortex; CSF: cerebrospinal fluid; DPFC: dorsal prefrontal cortex; FC: frontal cortex; HP: hippocampus; SEZ: subependymal zone. Red font indicates discrepancies among studies.
2.1. Microglia
Microglia are the resident brain innate immune cells that have been implicated in host defense against neurotropic pathogens, brain development, and neurodegenerative disorders [12]. The growing importance of these cells in behavioral illnesses is also highlighted by the growing attention they have received in neuroimmunological investigations studying possible alterations in their distribution and function in SCZ. Given the difficulty of sampling live human microglia, cell characterization in SCZ has been mostly conducted in post mortem brain samples. One of the earliest studies of microglia in SCZ was an analysis of embryonic microglia derived from female patients with SCZ in whom these cells displayed a highly phagocytic phenotype compared to healthy controls (HCs) with no psychiatric illnesses [13]. Subsequently, morphologically activated microglia have been observed in the prefrontal cortex (PFC) and visual cortex of paranoid and chronic patients with SCZ in close proximity to dystrophic oligodendrocytes [14,15,16]. Further subcategorization of patients with SCZ revealed that this abnormal microglia activation phenotype might contribute to oligodendrocyte dystrophy in schizophrenia patients with positive symptoms [17,18], providing some of the first morphological evidence for the possible involvement of microglial activation in the development of these SCZ-associated pathologies.
In addition to these morphometric studies, others attempted to localize activation markers on microglia by immunohistochemical analysis and found increases in HLA-DR+ activated microglia in the frontal/temporal cortex and the hippocampus in patients with SCZ [19,20]. Of note, these activated microglia exhibited some degenerating features [21] and were reportedly associated with interleukin IL1β expression in the PFC [22]. Microglia activation was also observed in some of these brain regions in patients with Alzheimer’s disease (AD) and affective disorders [23,24], suggesting the possible existence of microglia reactivity against a common dysfunctional neuronal circuit among various CNS disorders. However, microglia activation in SCZ and affective disorders remains to be validated as some studies failed to detect changes in HLA-DR+ microglia in various brain regions, including PFC, anterior cingulate cortex (ACC), and hippocampus, and/or attribute this microglia activation profile to death by suicide [25,26]. Besides HLA-DR expression, a unique microglial proteome might exist in SCZ. For example, S100 calcium-binding protein (S100) A8/A9 expression [27], an inflammatory marker, was found to be upregulated in frontal cortex microglia, while quinolinic acid expression, a neuroprotective molecule, was suppressed in CA1 hippocampal microglia in patients with SCZ [28].
2.2. Other Immune Cell Types
Besides microglia, abnormalities in other immune cells have also been detected in CNS samples of SCZ patients(). For example, dynamic trafficking of adaptive immune cells in the CNS has been linked to SCZ. Whole brain immunohistochemical quantitation of T cell and B cell frequencies showed marked increases in these lymphocytes in patients with SCZ and affective disorders compared to HCs [52]. Spatial analyses also revealed region-specific alterations of these lymphocytes in SCZ brain tissues. In this regard, immunohistochemical analysis in the dorsal PFC (DPFC) revealed a reduction in CD3+ T cell density in the leptomeningeal space of subjects with SCZ compared to HCs and no significant difference in the frequencies of these lymphocytes in the gray matter of both groups.
Monophagocyte-related alterations have also been reported in SCZ brains. In the neurogenic subependymal zone (SEZ), an SCZ subgroup with high inflammation (HC) (defined by elevated expression of IL-1β, IL-1R1, serine protease inhibitor member 3 [SERPINA3], and c-x-c motif chemokine ligand 8 [CXCL8] mRNA transcripts) showed higher expression of the identity markers of macrophages (CD163) and monocytes (CD14) than high-inflammation HCs [53]. Notably, increased infiltration of monophagocytes into the SEZ appeared to be a shared pathological feature between patients with SCZ and patients with BD [54]. In the mid brain, immunostaining revealed that CD163+ macrophage density was elevated in high-inflammation SCZ compared to HCs [55].
Nevertheless, the exact role played by these brain immune cells in SCZ remains contentious. For instance, immunohistochemical analysis in the DPFC of SCZ patients yielded no evidence of CD163+CD206+ perivascular macrophage infiltration into the brain parenchyma [33]. In contrast, a different study that transcriptionally quantified CD163 mRNA expression in the DPFC showed that macrophage accumulation in this brain region was a signature of high-inflammation SCZ [56].
Besides post mortem brain studies, cerebrospinal fluid (CSF) analysis of adaptive and innate immune cells in SCZ is another approach that has been investigated. For example, acute psychotic symptomatology in SCZ patients [57] was associated with an accumulation of monophagocytes in CSF samples. Further analysis revealed that this signature of innate immune alteration was accompanied by an increase in the frequency of lymphocytes with an activated phenotype during psychosis onset in SCZ [58].
3. Alterations in Circulating Immune Cells in SCZ
3.1. Monocytes
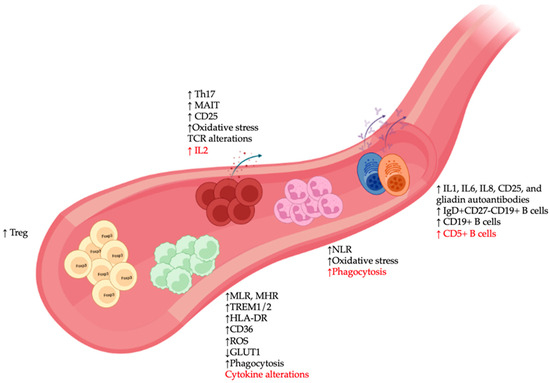
In the innate immune system, monocytes represent the counterparts in the circulatory system of microglia and are known for their plasticity in responding to environmental changes. There is a growing body of clinical evidence of monocyte alterations in SCZ blood samples [62] (Figure 2), including higher total monocyte counts in SCZ during the first episode of psychosis, although some discrepancies remain as to whether these alterations are linked to disease severity [63,64]. A similar increase in total monocyte number was also observed in patients with non-affective psychosis [65], while elevated counts of classical monocytes and proinflammatory monocytes have been linked to clozapine-treated and recent-onset SCZ, respectively [66,67].

Figure 2. Peripheral immune alterations in patients with schizophrenia (SCZ). Major changes in immune cell types in blood samples of SCZ patients included: (1) Alterations in monocytes such as increased monocyte-to-lymphocyte and monocyte to HDL ratios (MLR and MHR), changes in expression of various immunometabolic markers (TREM1/2, HLA-DR, CD36, reactive oxygen species [ROS], GLUT1), and abnormalities in phagocytosis and cytokine production; (2) elevated expression of various neutrophil-associated markers such as neutrophil to lymphocyte ratio (NLR), oxidative stress, and phagocytosis; (3) presence of various autoantibody-producing pathogenic B cell clones, as well as increased numbers of different B cell subsets; and (4) increased activation profile of T cells (CD25), alterations in oxidative stress and T cell receptor [76] repertoire, and accumulation of immunoregulatory T lymphocyte populations, such as regulatory T (Treg), IL17-producing T-helper (Th17), and mucosal-associated invariant T (MAIT) cells. Red font indicates discrepancies among studies.
These observations of alterations in various monocyte features represent a significant breakthrough in SCZ research. While many studies inferred a monocyte-associated gene set from bulk immune cell transcriptome profiling, the studies mentioned above typically focused on a phenotypic and functional characterization of the monocytes themselves. For example, an interferon gene signature in isolated monocytes was observed in SCZ, with dynamic changes over the disease course [77].
Of potential clinical utility is the presence of various monocyte-specific markers for treatment response monitoring and differential diagnosis of SCZ. Specifically, reduced glucose transporter (GLUT1) expression in monocytes has been proposed as a key diagnostic feature to distinguish SCZ from BD, MDD, and autistic spectrum disorder [89], while soluble CD14, an identity marker of circulating monocytes, could accurately predict subsequent SCZ diagnosis [90]. A monocytic transcription signature was also proposed as a candidate marker for monitoring beneficial simvastatin response in patients with SCZ [91]. The effectiveness of other antipsychotics, such as haloperidol/perazin and clozapine, can also be predicted by a reduced monocyte production of I-1/TNF-α and reactive oxygen species (ROS), respectively, while the effectiveness of olanzapine could be monitored by pre-treatment monocytic expression of the fatty acid receptor CD36 [83,92,93].
3.2. Granulocytes
Circulating granulocytes consist of three major myeloid cell subsets, namely basophils, eosinophils, and neutrophils. While the first two are rarely discussed in the context of neuropsychiatric illnesses, an increase in neutrophil-related parameters represents one of the most consistent findings regarding changes in peripheral immune cells in SCZ (Figure 2).
3.3. Natural Killer Cells
NK cells are a type of immune cell with both adaptive and innate features. They have been implicated in a wide range of human diseases, ranging from infection and cancer to CNS disorders. Potential abnormalities in both NK cell count and function have been documented in SCZ, although with significant discrepancies among studies. Flow cytometric analysis showed increased counts of NK cells in clozapine-treated chronic SCZ blood samples compared to HCs [67]. In contrast, computational deconvolution based on gene expression yielded lower NK cell numbers in both drug-naïve and medicated SCZ patients [125,126], and this decrease was uncorrelated to psychotic relapse/remission. A different flow cytometric study confirmed these lower NK cell counts in chronic SCZ; however, medication appeared to increase NK cell numbers [127]. These differences could be due to the different quantification methods used (flow cytometry vs. gene expression, whole blood vs. peripheral mononuclear cells). Chronic SCZ subpopulations who received differing regimens of antipsychotic drugs may exhibit distinct NK cell profiles. In fact, an earlier study involving a heterogenous SCZ cohort (more than four subtypes and treatment modalities) failed to detect any abnormalities in immune cell counts in blood samples [128]. Regarding the function of NK cells in SCZ, there have been conflicting reports on NK cell cytolytic activity, possibly related to significant variations in general NK cell lytic function, medication regimes, and SCZ subtypes [129,130,131,132]. With regard to phenotypes, some studies suggested that an elevated expression of NK-cell-activation markers, such as HLA-DR and natural killer group 2C (NKG2C), might be associated with the first episode of psychosis in SCZ patients compared to HCs.
3.4. B Lymphocytes
Considering that various autoantibody types have been found to be elevated in serum and CSF samples of SCZ patients, B cells, as producers of antibodies, have long been implicated in the autoimmune hypothesis of SCZ (Figure 2). For instance, several small-cohort studies suggested that autoantibodies may act against anti-glutamic acid decarboxylase (GAD), γ-aminobutyric acid A receptor 1 (GABAR1), anti-acetylcholine receptor (A7ChR), and N-metil-D-aspartato receptor (NMDAR) in SCZ pathogenesis [136,137,138,139]. However, large-scale studies (n > 150) failed to confirm the biological significance of these autoantibodies in this disorder [140,141,142], or they detected only a small range of autoantibodies in low concentrations in certain SCZ subsets, such as clinically high-risk SCZ, SCZ with the first episode of psychosis, and SCZ with tardive dyskinesia.
3.5. T Lymphocytes
Numerous phenotypic studies of various circulating T cell subsets have been conducted in SCZ (Figure 2). While the findings were inconsistent with regard to the distribution of total CD3+ T cells, CD4+ helper T cells, and CD8+ cytotoxic T cells [127,156,160,161,162], they mostly agreed with regard to increased T cell activation [66,163,164]. Furthermore, activated T cells in medicated SCZ patients appeared to have higher levels of CD25 than drug-naïve patients. Along with this ex vivo-activated phenotype, several studies observed a reduced responsiveness to in vitro stimulation of IL-2 production in T cells from drug-naïve SCZ patients compared to HCs [165,166,167]. However, this decrease in IL-2 production was not observed in a study of paranoid and residual SCZ [99], possibly due to how patients were stratified in this study and/or the use of purified T cells/peripheral blood mononuclear cells vs. whole blood for in vitro assays. Regarding T-helper (Th) cell subtypes, an increase in Th2 cells was reportedly associated with the SCZ subtype with a pro-inflammatory monocyte feature [66]; however, this alteration could not be confirmed by a different study [99]. The same two studies also yielded inconsistent findings regarding the role of Th1 cells. In contrast, consensus exists regarding the elevated numbers of regulatory T cells (Treg) and IL-17-producing Th cells (Th17) in SCZ [66,135,163,168,169,170].
4. Implications for Mechanistic Studies and Therapeutic Development
To explore disease mechanisms of SCZ, various in vitro and animal studies have been established, with induced pluripotent stem cells derived from SCZ patients and rodent strains based on human genetics as the most clinically relevant attempts to model SCZ-associated pathology [178,179]. However, most immune-related studies to date have focused exclusively on the role of microglia, but not other peripheral immune cell subsets, in this psychiatric illness.
Anomalies in various immune cell subsets and their trafficking patterns in SCZ also suggest the potential utility of cell-type-specific immunotherapy as a novel pharmacological approach for this psychiatric illness [184,185]. In this regard, immunomodulators aiming to inhibit lymphocyte trafficking (fingolimod) and deplete B cells (anti-CD20 monoclonal antibody, rituximab) could significantly improve negative symptoms and general psychopathology of SCZ, respectively [186,187].
Besides these classical immunotherapies, the potential clinical efficacy in SCZ of other anti-inflammatory treatments, including aspirin, minocycline, N-acetylcysteine, estrogens, telmisartan (an angiotensin receptor 1 antagonist), and pioglitazone (a PPAR-γ antagonist) [193,194,195], has been observed. Interestingly, other anti-inflammatory medications, such as celecoxib, davunetide, dextromethorphan, fatty acids, pregnenolone, statins, and varenicline, did not have a significant impact on SCZ symptoms in a recent meta-analysis, highlighting the presence of inflammatory-pathway-specific abnormalities in SCZ development.
This entry is adapted from the peer-reviewed paper 10.3390/cells12162099
This entry is offline, you can click here to edit this entry!
