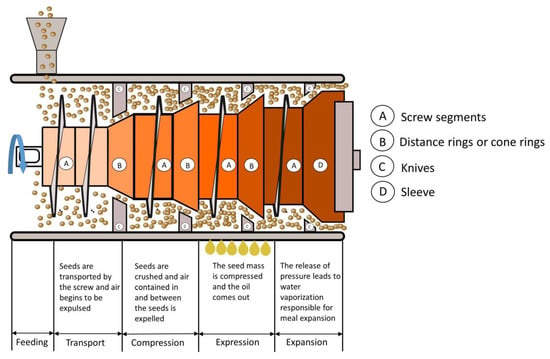
The pressing process is generally divided into pre-pressing or full pressing of the oilseeds. Pre-pressing yields about 60% of the available oil, while full pressing affords almost 90%. Oily materials with more than 30% oil by weight tend to decompose in the extractor after most of the oil has been extracted, resulting in poor final extraction and high solvent retention. Solvent extraction is most effective when the oily material contains about 20% of oil. In this condition, the system is in thermodynamic equilibrium, as the waste heat from desolventising of the meal is sufficient to serve as the main heat source for evaporation of the solvent in the miscella. Therefore, oily materials with an oil content of more than 30% (e.g., rapeseed/canola seeds, sunflower) are usually reduced to 20% by pre-pressing prior to solvent extraction.
2.2. Oilseed Solvent Extraction
2.2.1. General Process and Parameters
The solvent extraction process consists of five closely related subprocesses: solvent extraction, meal desolventisation, drying and cooling of the meal, distillation of the miscella and solvent recovery. Extraction is always used when a residual oil content of <2% is desired. Most vegetable oils are obtained by solvent extraction.
The solvent extraction process has the advantage of a much higher oil yield (<1% residual oil in the meal) than mechanical extraction, as well as lower operating costs per unit. However, solvent extraction plants have high initial costs, not least because the entire plant must be explosion-proof. Typically, these plants process between 1000 and 5000 tonnes of seed per day, with construction costs ranging from USD 15 million to USD 75 million.
Ideally, solvents used for the extraction of vegetable oils should show high oil solubility at low temperatures, high selectivity for lipid compounds, chemical inertness (non-explosive), non-toxicity, low viscosity and surface tension, low boiling point and low heat of vaporisation, and they should be environmentally friendly. Obviously, no extraction solvent can fulfil all the properties from the above list. Therefore, the best compromise must be found based on priorities. Before hexane, carbon disulphide, benzene and trichloroethylene were used, which were later banned because of their high toxicity
[5].
The solvent exploited in most oilseed extraction plants around the world is hexane, a mixture of saturated acyclic hydrocarbons with six carbon atoms that distils between 64 °C and 70 °C
[14]. Commercially available hexane contains about 65% of
n-hexane. As well as this main component, hexane can also contain other hexane isomers, cyclic hydrocarbons such as cyclohexane or even aromatics such as toluene or benzene
[15]. The low viscosity of hexane is favourable for extraction, especially for percolate extraction. The viscosity of hexane decreases almost linearly from 0.4 cP (0 °C) to 0.37 cP at 15 °C. Between 15 and 25 °C the curve is steeper and then returns to an almost linear curve up to 50 °C (0.25 cP).
The oil content in the mixture influences the vapour pressure and leads to different boiling points. Hexane vapours can form an explosive mixture with air, so special care must be taken when building and operating solvent extraction plants. Hexane/air mixtures are explosive in the range of 1.2 to 7.4% v/v hexane. Hexane extraction allows high oil recovery at low production costs. In addition, the meal defatted with hexane is odourless and has a low residual oil content, which makes it very marketable.
During solvent extraction, the miscella diffuses through the cell walls to the oil bodies inside the cells. As the miscella continues to penetrate and dissolve the oil, the pressure in the cell increases and the concentrated miscella diffuses back out of the cell. Once the more concentrated miscella reaches the miscella bath, the concentration of the bath gradually increases. This process continues until the miscella concentration in the cells of the oil material reaches equilibrium with the concentration of the miscella bath
[3].
Six parameters affect the performance of solvent extraction systems: (1) contact time, (2) particle thickness, (3) number of extraction stages, (4), miscella flow rate, (5) extractor temperature and (6) solvent retention
[5].
The extraction time needed depends on the kind of seed, its pre-treatment and the equipment used. Adequate contact time (1) is critical to maximise extraction efficiency. The residence time corresponds to the time the oily material spends in the extractor. The residence time can be divided into wash time and drain time. This latter is the time during which the oily material drains before being emptied, while the washing time includes the contact time and the dormant time. Extraction only takes place during the contact time, which is the time the oily material is in contact with the miscella. The dormant time is the time an oily particle spends in the wash zone of the extractor where it is not in contact with the miscella. The ratio between contact and dormant times varies depending on the extractor. Extractors with a deep bed of material and a small surface area generally operate by immersion and provide a very high contact to dormant times ratio. Extractors with a shallow bed depth and a large bed surface area generally work by percolation and offer a lower contact to dormant times ratio. Despite the higher initial investment, the long-term benefits of a longer contact time often justify oversizing the extractor. In the commercial operation of a shallow or deep bed extractor, the residence time for each extraction stage varies between 7 and 10 min or an average of 45 min for the entire extraction process.
The structure of the oilseeds (2) at the entrance of the extractor depends on the cell structure of the material, but especially on the preparation steps. For almost all oil materials, the preparation ends with flaking, which reduces its thickness and thus the distance and number of cell walls that the miscella must pass to reach the oil bodies. Depending on the structure of the oil material, the time needed for the miscella that enters the cells to reach equilibrium with the miscella outside changes. For example, soybean flakes have a cell structure that allows them to reach equilibrium in about 5 min each time the extractor is filled, while sunflower cakes take about 9 min and rapeseed cakes about 12 min. If all other extraction parameters remain constant, a smaller extractor can be used by reducing the particle thickness. However, a further reduction in particle thickness involves additional costs. For all oily materials, the economic balance between the initial cost of the extractor and the continuous electricity cost required for flaking can be analysed and the optimal particle thickness determined
[3].
An extractor with only one miscella stage would require a large amount of solvent to achieve high extraction efficiency. The energy required to evaporate the solvent in the mixture would be enormous; therefore, multi-stage countercurrent extractors are used. By iterating the mass balance, the minimum number of stages in the mixture can be calculated for a given solvent/material ratio. For an energetically competitive distillation system, the solvent/material ratio must be kept close to or below 1. Theoretically, the higher the number of stages in the mixture, the higher the extraction efficiency. However, if the individual stages do not have enough contact time to reach equilibrium, the addition of stages will not further reduce the residual oil. In this case, more extraction stages simply mean more pumping and more potential solvent losses. The number of extraction stages (3) is generally determined by the total time of the wash zone and the number of stages that can theoretically reach equilibrium within the time of the wash zone. Most commercially available extractors deal with between five and nine extraction stages
[3].
Miscella flow rate (4) is the maximum volumetric flow rate of miscella that can flow through the material bed per unit area of it (m3/h per m2 of material bed). The main causes of reduced miscella flow are the presence of thinner than normal flakes, high surface moisture or an abundance of fine particles. A uniform shape of the material arriving at the extractor ensures uniform miscella flow rates.
Increasing the temperature in the extractor (5) increases the diffusivity of the miscella through the cell walls of the oil material. To optimise extraction, it is therefore necessary to reach the highest possible temperature. However, the temperature influences not only the extraction rate but also the proportion of non-oil lipids (e.g., non-hydratable phospholipids) and non-lipid components in the crude oil
[16]. The maximum operating temperature of the extractor depends on the safe working temperature of the solvent. Since the boiling range of commercial hexane at sea level is usually 64–70 °C, the highest possible temperature to avoid boiling is 63 °C. However, most extractors work at 60 °C to ensure a higher safety margin. To avoid heat loss, the oil transport system and the extractor are thermally insulated.
After the washing area of the extractor, the miscella retained by the extracted material is drained by gravity (6). This gravity drainage usually takes between 5 and 20 min and varies depending on the depth of the material bed in the extractor. The deeper the material bed, the longer the drainage time required. After drainage, meal from flaked oilseeds usually retains 30–40% by weight of solvent (hexane). Pre-pressed cake and oilseeds prepared by extrusion can free drain to 20–25% solvent before leaving the extraction vessel
[9]. The more solvent retained in the meal, the more energy is required for the desolventisation step and the higher the risk of protein degradation. Solvents with higher polarity than hexane, especially in the presence of water, are better retained in the meal and are more difficult to be removed, resulting in higher energy consumption and a greater loss of protein solubility
[17]. In the meal desolventisation system, the solvent is evaporated, leaving traces of oil, often referred to as residual oil. This contains high concentrations of phosphatides (about 20%) and other non-triglyceride compounds
[5]. Adequate draining time of the extractor is the most economical way to minimise the retention of miscella.
To reduce operating costs, it is crucial to maximise the oil concentration in the miscella at the outlet of the extractor, reduce the retention of solvent in the wet meal and achieve the lowest effective solvent to solid ratio that results in acceptable residual oil in the meal
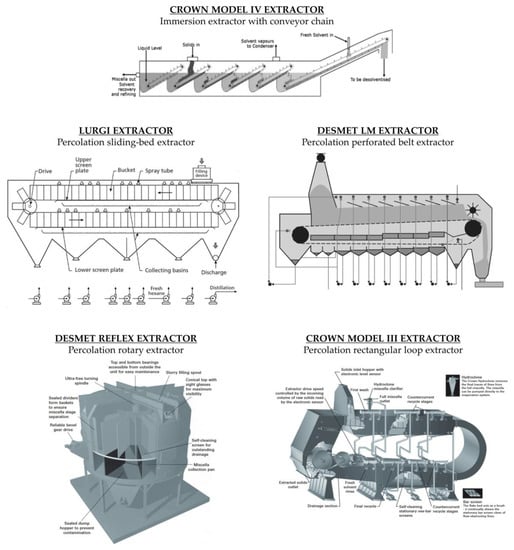
[18]. Industrial solvent extraction plants are divided into batch extractors and continuous extractors. The latter follow the principle of countercurrent extraction and are divided into immersion extractors or percolation extractors. Some countercurrent extractors are shown in
Figure 2.
Figure 2. Countercurrent immersion and percolation extractors
[3][9][12][19].
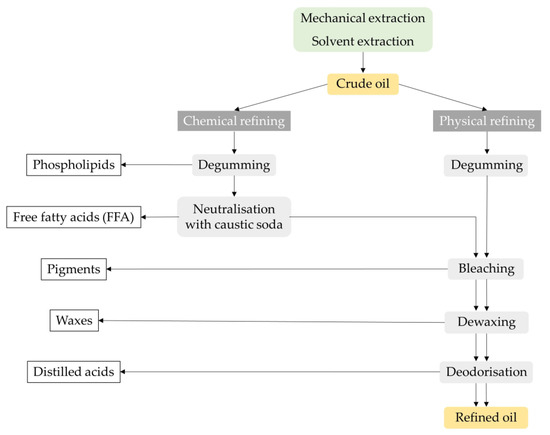
Except for virgin oils, crude oils cannot be consumed directly or used in various foods without technological refining processes. Crude oils such as soybean, rapeseed, palm, corn and sunflower oils must be purified or refined before consumption. The aim of refining is to obtain an odourless and rather neutral-tasting oil that is limpid and colourless and free of contaminants
[20]. Compounds known to have a negative impact on the quality and stability of oils include free fatty acids, unsaponifiables, waxes, pigments, solid impurities (especially fibres) and oxidation products (peroxides, aldehydes, ketones and oxidised fatty acids). In addition, vegetable oils may contain some contaminants: pesticides, trace metals, mineral oil aromatic hydrocarbons (MOAH), aflatoxins, dioxins, polycyclic aromatic hydrocarbons (PAH) and traces of organic solvents. However, one of the main disadvantages of refining is the loss of substances responsible for the healthy and technological properties of the oils, such as tocopherols, phospholipids, squalene, polyphenols and phytosterols
[21]. The two main industrial processes for vegetable oil refining are chemical and physical refining, as described in
Figure 3.
Figure 3. Crude oil chemical and physical refining process: a general overview.
The difference between these two processes lies in the method used to remove the free fatty acids. In chemical refining, the free fatty acids are removed by adding caustic soda and separating the soap by centrifugation (mechanical separation), while in physical refining, the free fatty acids and other compounds are removed in the final step by distillation under a high vacuum with steam injection
[20].
2.2.2. Batch Extractors
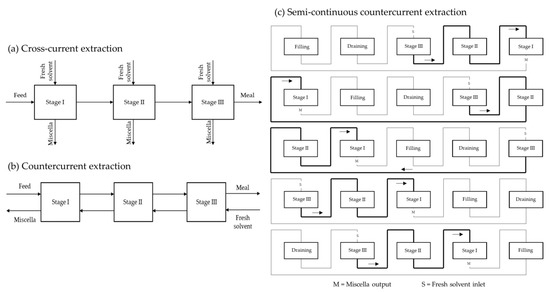
Early industrial plants used batch extractors, which remained the only devices for solvent oil extraction for several decades. Batch extractors are simple systems with a seed inlet and a meal outlet. The extraction occurs through maceration, with the extraction solvent being pumped into the extractor, which is usually equipped with an agitator. At the end of the extraction, the miscella is drawn off and the meal is desolventised in the extraction vessel by indirect steam heating, sometimes combined with a live steam injection. The resulting vapours leave the vessel through the ventilation system and are fed into the condenser. Batch extraction is no longer used in large-scale plants, while it continues to be used for small production such as the extraction of specialty oils. Some disadvantages of batch extractors can be overcome if several batch extractors are combined into one group extractor (
Figure 4)
[22].
Figure 4. (a) Cross-current extraction principle; (b) countercurrent extraction principle; (c) semi-continuous countercurrent extraction.
In this way, a semi-continuous process is achieved that also allows countercurrent extraction. With this configuration, each time a batch of miscella is discharged from one extractor, it is pumped to another extractor containing material previously extracted with an oil-richer miscella. Stage I contains the raw seed and Stage II contains extracted seed, whereas Stage III contains the furthest extracted seed. The extraction solvent is pumped from III to I countercurrent. The same principle of successive extraction cycles in continuous countercurrent extractors is obtained. An additional vessel for feeding fresh seeds allows for a semi-continuous system (Figure 4c).
2.2.3. Countercurrent Continuous Extractors
In the immersion process, the seed is completely immersed in the solvent. The static system requires stirring to ensure the exchange of the locally concentrated solvent. Immersion extractors are particularly suitable for the extraction of fibre-rich oilseeds. An example of a countercurrent immersion extractor is the Crown Model IV (
Figure 4). This extractor model is designed for the immersion extraction of powdery materials that cannot be extracted in percolation extractors. In this device, the material itself moves through the successive solvent tanks by means of an inclined conveyor that slowly draws the still immersed material into the solvent pool. The gentle movement and rotation of the bed minimises the formation of fines. The solids rise above the solvent level as the inclined conveyor rotates around the top pulley, and then fall freely through the solvent into the second pool. The densities of the material and the solvent must be sufficiently different to allow the particles to fall. This process is repeated for all the extraction stages. Above the last inclined ramp, fresh solvent is added, and the mixture is fully aspirated at the point where the solids enter the extractor. The last inclined ramp allows the solvent retained by the material to drain off before it leaves the extractor
[19].
The percolation process is based on the principle of permanent wetting of the surface by percolating solvent. During percolation, there is a constant exchange between the free-flowing solvent and the solvent trapped or absorbed by the material. This phenomenon ensures that locally oil-rich solvent is permanently replaced by fresh or low-concentration solvent. The process requires an effective pre-treatment of the seed to obtain as many open cells as possible. Compared to immersion, the material does not have to be stirred up, thus avoiding a further undesirable reduction in size. In addition, only a limited portion of the fine particles mix with the miscella, as the material acts as a filter and retains them. In optimised countercurrent percolation systems, a miscella oil content of 30% can be achieved
[22]. There are different types of percolation extractors: rotary, perforated belt, sliding-bed and rectangular loop extractors.
In rotary extractors there are usually five stages of extraction, followed by a sixth one where fresh hexane is used for the final wash. Ideally, there should be a slight preponderance of liquid over solids in each stage. This ensures that all solids are in contact with the liquid. If the solids bed allows solvent to flow too quickly so that no liquid column can be maintained, the solvent/mixture must be evenly distributed over the material. The preparation of the material must allow adequate percolation flow. A too slow flow may result in flooding of the cell with the mixture overflowing into the next one. The presence of channels in the bed of an inhomogeneous material can divert the solvent flow via the path of least resistance. Usually, in a deep bed extractor, flooding is more likely than too rapid percolation. Extrusion of the material can be useful as it agglomerates the fines and creates a more uniform bed of material
[5]. To achieve good contact between the matrix and solvent, some models of deep bed extractors are equipped with a chamber in which the material is immersed in the solvent before extraction. This process reduces the risk of channelling during the percolation, where the solvent flows along preferential channels and does not adequately contact certain areas of the bed. Shallow bed units are usually loaded dry. The most common rotary extractor supplied today is the Reflex Extractor (Desmet Ballestra Group, Paris, France), where the material is mixed with the miscella and fed into the rotating baskets. This design constantly reduces the transport of solvents in the spent material to the desolventiser, saving steam energy
[23].
In a perforated belt extractor, the material bed is a continuous mass extending the length of the extractor. In these systems, percolation occurs through an endless loop perforated belt that keeps the bed of material moving. The belt transports the incoming material through the extractor stages, each consisting of a sump below the belt and miscella spray heads above. The residence time is adjusted depending on the belt speed. The belt is slightly inclined. This helps the miscella to flow countercurrent through the material more efficiently. The incoming material, mixed with concentrated miscella, is deposited on the belt to form a bed of material. The depth of the bed can vary but is usually in the middle range (1.5–2.0 m deep). As with the other extractor types, there are several extraction stages and a final wash with fresh hexane. Each miscella pump returns the miscella to the same stage from which it came. The material moves countercurrent to the miscella that overflows from one sump chamber to the previous one. The most common perforated belt extractor supplied today is the LM™ Extractor (Desmet Ballestra Group, Paris, France).
A sliding-bed extractor pushes the solids along a fixed steel plate with a special groove that allows the miscella to pass through while the materials are retained. The depth of the material bed can be adjusted during operation and is usually between 0.5 and 1.3 m. The extractor consists of two overlapping plates. The moving cell assembly wraps around a drive pulley, causing the oilseed contents to be turned over and pushed onto a second perforated plate, then making a second pass through the extraction chamber. Countercurrent miscella washes are introduced onto the bed of cells as they pass under miscella spray heads
[9]. Sliding-bed extractors process flaked material, extruded collets or prepressed cake
[5]. The most common sliding-bed extractor supplied today is the Lurgi Extractor (JJ-Lurgi Engineering, Selangor Darul Ehsan, Malaysia).
In rectangular loop extractors, the material is drawn through a closed ring-shaped chamber. The housing closes around itself in a spiral. The solid material is deposited in a shallow bed, usually less than 1.0 m, and travels a distance equivalent to 50 times the depth of the bed
[5]. Fresh oilseeds are conveyed through an inlet to the upper level and sprayed with a rich mixture. The oilseeds undergo a multi-stage (usually seven) countercurrent extraction as they pass through the ring. External valves allow each pump to return part of the miscella to the stage from which it came and part to the previous stage. Sometimes the entire miscella is recycled to the stage from which it came to extend the contact time and maintain a sufficient liquid height above the material. The final wash occurs using fresh solvent, followed by a dripping period of up to about 30% solvent by weight before the extracted material comes out
[9]. The most common rectangular loop extractor supplied today is the Crown Type III Extractor (Crown Iron Works, Minneapolis, MN, USA).
2.2.4. Conventional Solvent Extraction
Hexane is a petrochemical solvent used for the extraction of vegetable oils and also to produce flavours and fragrances, natural extracts, pharmaceuticals and food supplements. The global demand for hexane in these sectors is about 1.1 Mt per year, of which 650 Kt are used for oilseed extraction and 450 Kt to produce natural extracts and specialty oils
[15]. In recent years, a shift in consumer awareness has been observed worldwide, reflecting growing concern about the health and sustainability of production systems. The same trend is also reflected in recent policies, in particular in the European Union (EU), with the adoption of the European Green Deal in 2020, which aims at climate neutrality, a circular economy and a toxic-free environment. In this context, the new Safe and Sustainable-By-Design (SSbD) standard aims to ensure that chemicals, materials and products are designed, manufactured and used in a way that does not harm humans and the environment
[24].
Among the solvents exploited at industrial level for the extraction of non-polar edible natural products such as dyes, flavours, fragrances or lipids, hexane is undoubtedly the most used, and it appears on the list of extraction solvents allowed for foods or food ingredients production in the EU (Directive 2009/32/EC)
[14]. This substance has been known for more than fifty years for its neurotoxicity and reproductive toxicity, but new evidence has shown that hexane is also a potential endocrine disruptor.
n-hexane (the main isomer of hexane) is classified as STOT RE 2 under the Regulation on Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), which means that it is suspected of causing organ damage through prolonged or repeated exposure. Currently, a process is underway to reclassify
n-hexane from STOT RE 2 to STOT RE 1, i.e., from suspected to proven to be neurotoxic to humans.
Researchers presented applications of hexane in food products and ingredients’ extraction and evaluated new evidence of its toxicity. Finally, some alternatives to hexane for the extraction of natural products were listed
[15]. At present, research and development of environmentally friendly and safe extraction methods is essential for the gradual replacement of toxic solvents such as hexane. Green solvents are supposed to be non-toxic or low-toxic, safe to use and handle, effective and derived from renewable resources. They generally meet some of the 12 principles of Green Chemistry established by Anastas and Eghbali
[25] and the 6 principles of Green Extraction established by Chemat et al.
[26].
Directive 2009/32/EC contains a first list of solvents for which no conditions are specified, and a second list for which permitted uses and maximum residue limits are set. Among the solvents listed in Directive 2009/32/EC, five of the seven solvents on the first list are compatible with oil extraction and are therefore potential alternatives to hexane. These include butane, ethyl acetate, ethanol, carbon dioxide and acetone
[27]. Of these five solvents, ethanol has the most extensive research literature.