Chemotherapy causes substantial thinning or loss of hair, termed chemotherapy-induced alopecia (CIA), in approximately 65% of patients. CIA is ranked as one of the most distressing adverse effects of chemotherapy, but interventional options have been limited. Here we discuss scalp cooling, the only FDA-cleared method, and other options being tested, to prevent CIA.
- scalp cooling
- chemotherapy-induced alopecia
1. Introduction
Globally, 18.1 million people were diagnosed with cancer (excluding non-melanoma skin cancers) in 2020, and this number is projected to rise to 26 million in 2040 [1,2]. By 2040, the number of patients requiring first-course chemotherapy each year will increase by approximately 50% to 15.0 million [2]. Among patients receiving chemotherapy, an estimated 65% are expected to develop chemotherapy-induced alopecia (CIA) and possible changes in hair pigmentation, texture, quantity, and growth [3,4]. While many common targeted therapies and some immunotherapies are classified as chemotherapy in the National Cancer Database (NCDB), the current discussion focus on CIA induced by cytotoxic drugs [5].
CIA is one of the most visible and dreaded adverse effects of systemic cytotoxic drugs for both male and female patients [4,6,7]. Cytotoxic drugs kill rapidly proliferating cells, not only the cancer cells but also highly proliferative healthy cells such as hematopoietic cells, intestinal epithelial cells, and hair follicle (HF) keratinocytes during the growth phase called anagen. Within the anagen HF, the main targets of cytotoxic drugs are the highly proliferative keratinocytes in the hair matrix at the bottom of the HF and their pigmentary system, leading to rapid apoptosis and hair shaft breakage/shedding [8]. Because up to 90% of scalp HFs are in the growth phase at any given time, scalp hair loss is often massive within weeks of the initiation of chemotherapy [9]. The risk of CIA and the degree of hair loss differ substantially based on the medication, dose, frequency, duration, and route of administration. The incidence of CIA is more than 80% for microtubule-stabilizing agents (e.g., paclitaxel), 60–100% for topoisomerase inhibitors (e.g., doxorubicin), more than 60% for alkylators (e.g., cyclophosphamide), and 10–50% for antimetabolites (e.g., 5-fluorouracil and leucovorin) [10]. Combination therapy can further increase the incidence. Beard, eyebrow, and eyelashes can also be affected, and the severity of these hair defects may vary by the specific drugs and treatment regimens [4,7]. While alopecia is generally reversible 3–6 months after cessation of chemotherapy (the new hair may show different texture and color), some patients experience no hair regrowth or only partial hair regrowth. Permanent CIA (pCIA) is diagnosed when there is no hair regrowth or incomplete hair regrowth 6 months after chemotherapy cessation. The incidence of pCIA is dose- and schedule-related and has been associated with docetaxel given at doses of 75 mg/m2 or higher per cycle, and less commonly paclitaxel [11,12,13,14]. In a study of 383 patients treated for breast cancer in the UK, pCIA was reported by 23.3% of patients receiving docetaxel (among a total of 245 patients) and 10.1% paclitaxel (among a total of 138 patients) (p < 0.01) [13]. In a study of 61 patients treated for breast cancer in Korea, the incidences of pCIA at 6 months and 3 years were 39.5% and 42.3%, respectively [12]. As a result, pCIA (together with vision damage) is at the center of thousands of lawsuits against docetaxel (sold under Taxotere) manufacturer Sanofi-Aventis. Depletion of HF keratinocyte stem cells via apoptosis, DNA damage, and epithelial-mesenchymal transition were detected upon chemotherapy in human scalp HF organ culture and may be one of the underlying mechanisms of pCIA [15,16].
Given that scalp and facial hair are key elements of good health, beauty, and youth in social communication [17], CIA has significant negative impact on patients’ self-esteem, body image, sexuality, and overall quality of life [4,7,8,18]. In a study of 179 male and female patients who developed CIA, 101 (56.4%) patients felt that alopecia was the worst side effect of chemotherapy, 129 (72%) patients said hair loss was affecting their social life, and 37 (20.6%) patients used hair accessories to hide the hair loss [6]. Cancer patients would even consider rejecting life-saving chemotherapy for fear of CIA [4,19]. Being a public sign of the illness, the visibility of alopecia makes it difficult for patients to keep their cancer status private; the moment alopecia becomes apparent is often the moment of public recognition of the patient’s cancer treatment [20]. Consequently, patients with CIA may begin to perceive certain changes in the attitudes towards them, ranging from sympathy to rejection [20]. There are excellent recent reviews on the clinical features, diagnosis, pathobiology of CIA, and CIA effects on quality of life [3,4,9,21,22,23,24].
Despite the large number of patients suffering from CIA and its severe negative impact, only one approach, i.e., scalp cooling, has been cleared by the US Food and Drug Administration (FDA, 2015) to prevent CIA. Encouragingly, small clinical trials and preclinical studies suggest calcitriol, Keratinocyte Growth Factor (KGF), topical vasoconstrictors, and low-intensity ultrasound may also prevent or reduce CIA. These preventative methods are discussed below.
2. Scalp Cooling Systems and Cold Caps
Scalp cooling has been used since the late 1970s to prevent CIA, and the scalp cooling system DigniCap was the first device cleared by the U.S. Food and Drug Administration (FDA) for the prevention of CIA in 2015 in breast cancer patients [9]. Scalp cooling prevents or reduces CIA via two main mechanisms: first, by reducing the scalp temperature using cold air, gel packs, or electronically cooled caps, local vasoconstriction reduces drug inflow to the scalp, consequently limiting the local concentration of chemotherapeutic agents in the HFs; second, the low temperature also helps to reduce cellular metabolism in the HFs, making them less vulnerable to the antimitotic and antimetabolic effects of chemotherapeutic drugs [20]. Additionally, low temperature-induced cell cycle arrest at the G0/G1 phase, increased HSP70 accumulation to protect cells from stresses, and reduced cell apoptosis have also been shown as potential mechanisms of HF protection against CIA [25].
Currently, there are several FDA-cleared scalp cooling systems available to patients, as well as cold caps that are not yet cleared by the FDA (Table 1). The cooling systems are mobile cooling units that pump circulating cold coolant to cool the scalp, with the temperature accurately controlled by a computer. They are operated by healthcare providers (Table 1). The cold caps use 3–8 caps containing coolant gels that can be re-frozen quickly. Temperature is carefully monitored, and the caps are changed manually. They are self-administered by the patient’s companion (Table 1). While some costs may be reimbursed by some insurance plans for the FDA-cleared cooling systems, others are not, and costs and access remain an important issue for many patients.
| Device | Date of FDA Clearance |
Device Description | Temperature Control by Computer |
Advantages | Adverse Effects |
Operator | Manufacturer | Costs |
|---|---|---|---|---|---|---|---|---|
| DigniCap C3 Scalp Cooling System |
2015 (for breast cancer) * | mobile cooling unit that uses circulating cold coolant | X | • The wrap is fitted at the beginning of treatment and remains on until completion • Starting from room temperature: comfortable |
feeling of coldness, headache, scalp pain and/or light-headedness, dizziness |
health-care providers administered |
Dignitana | ~USD 1500–2000 |
| DigniCap Delta Scalp Cooling System |
06/27/19 | Digitana | ~USD 1500–2000 | |||||
| Paxman Scalp Cooling System | 04/19/17, later expanded to solid tumors |
Paxman Coolers Limited | capped at USD 2200 | |||||
| Amma Cooler Heads cold cap | 12/08/21 | Cooler Heads | ~USD 2000 rental costs | |||||
| Penguin Cold Caps | not yet ** | 3-cap system: Crylon Gel in the caps | no | • Portable • Caps chill fast |
self-administered | Penguin Cold Caps |
USD 419/month rental costs | |
| Chemo Cold Caps |
not yet | 6-cap system: caps filled with coolant gel | Arctic Cold Caps LLC |
USD 379/month rental costs | ||||
| Arctic Cold Caps |
not yet | 8-cap system: caps filled with glycerin-based hydr0-gel that refreezes in 2 h | Arctic Cold Caps LLC |
USD 379/month rental costs |
To achieve effective protection, the scalp must attain a subcutaneous (1–2 mm) temperature below 22 °C, which is equivalent to an epicutaneous temperature of 19 °C, although greater preventive effects could be achieved with temperatures close to 15 °C [20,26]. Scalp cooling begins approximately 30 min before chemotherapy starts, continues during the infusion, and must continue for a set period after the conclusion of treatment. The post-infusion cooling time depends on the pharmacokinetics of the chemotherapeutic agents and doses used, typically 60–180 min. The cooling cap remains on the scalp for another 5–10 min to return to room temperature. Adverse effects such as a feeling of coldness, neck and shoulder discomfort, headache, scalp pain, forehead pain, nausea, and/or light-headedness and dizziness have been reported, but are temporary [27]. However, patient compliance is high, and efficacy of protection from hair loss by scalp cooling has been increasingly demonstrated over the past 20 years, with average success rates of 50–70% [27]. It is important to note that efficacy for hair preservation depends on chemotherapy type, e.g., higher efficacy is associated with a taxane-alone regimen, and much lower efficacy is associated with an anthracycline-based regimen [28]. Over 70% of current clinical trials aimed at prevention and treatment of CIA are testing scalp cooling devices, alone or in combination with other approaches (Table 2).
| Category | NCT Number |
Interventions | Completed | Recruiting | Not Yet Recruiting | Device | RCT, Parallel Assignment |
Single Group Assignment |
Open Label | Gender | Target Sample Size/Enrollment | Prevention | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scalp cooling | NCT01831024 | Dignicap System | * | X | Non-R CT | X | Female | 110 | X | ||||
| NCT03712696 | Device: DIGNICAP™ | X | X | X | X | Female | 139 | X | |||||
| NCT04630080 | Scalp cooling | X | X | X | X | Female | 100 | X | |||||
| NCT05213936 | Scalp cooling with hairstyle using conditioner and water emulsion | X | X | Non-R CT | Single blind (Investigator) | All | 30 | X | |||||
| NCT03248193 | Concomitant limb cryocompression and scalp cooling |
* | X | Non-R CT | X | All | 50 | X | |||||
| NCT04986579 | Paxman Scalp Cooling System | X | X | Non-R CT | X | All | 120 | X | |||||
| NCT01008774 | Paxman Cooling Machine; Cold Caps |
X | X | Non-R CT | X | All | 239 | X | |||||
| NCT04180579 | PAXMAN Scalp Cooler | ** | X | X | X | Female | 34 | X | |||||
| NCT04764357 | Paxman Scalp Cooling System | X | X | X | X | All | 40 | X | |||||
| NCT04117815 | Paxman Scalp Cooling System | X | X | *** | Female | 128 | X | ||||||
| NCT04626895 | Paxman Scalp Cooling Device | X | X | X | X | Female | 15 | X | |||||
| NCT05533320 | Paxman Scalp Cooling System | X | X | X | X | Female | 30 | X | |||||
| NCT04678544 | Paxman Scalp Cooling System 2 | X | X | X | Single blind (Outcomes Assessor) | Female | 170 | X | |||||
| NCT04168242 | Scalp cooling Paxman Orbis II system |
** | X | X | X | Female | 80 | X | |||||
| NCT01986140 | PAXMAN Orbis Scalp Cooler Treatment with Orbis scalp cooling cap | ** | X | X | Single blind (Care Provider) | Female | 236 | X | |||||
| NCT03289364 | Penguin Cold Caps | * | X | X | X | All | 9 | ||||||
| NCT05484973 | AMMA Portable Scalp Cooling System | X | X | X | X | Female | 125 | X | |||||
| NCT05365243 | AMMA Portable Scalp Cooling System | X | X | X | X | Female | 12 | X | |||||
| NCT03711877 | Scalp cooling system; chemical cold cap | X | X | X | X | Female | 256 | X | |||||
| PBMT | NCT05177289 | Theradome® LH80 pro combined with scalp cooling | X | X | X | Single blind (Outcomes Assessor) | Female | 72 | X | X | |||
| NCT04036994 | Photobiomodulation therapy | X | X | X | Single blind (Participant) | Female | 30 | X | |||||
| NCT05397457 | Low-level light therapy | X | X | X | X | Female | 88 | X | |||||
| Topical | NCT02919735 | Topical CG 428 herbal medicinal solution | X | X | Double blind (Participant, Investigator) | Female | 40 | X | X | ||||
| NCT02986412 | Topical CG 428 herbal lotion |
X | X | X | Female | 19 | X | ||||||
| NCT02605629 | Topical CG 428 herbal lotion |
X | X | Double blind (Participant, Investigator) | Female | 32 | X | ||||||
| NCT04554732 | Topical Keratinocyte growth factor | X | X | X | Female | 28 | X | ||||||
| NCT01588522 | Topical compound 31, 543 Calcitriol | X | X | X | All | 30 | X | ||||||
| Oral | NCT03831334 | Drug: oral minoxidil | X | X | X | All | 25 | X | |||||
| Injection | NCT04459650 | Platelet Rich Plasma | ** | X | X | All | 30 | X | |||||
| - | NCT02530177 | Clinical Assessment of alopecia and pCIA, skin aging and nail changes: Observational |
** | **** | Female | 546 | - | - |
While the FDA has cleared the expanded use of scalp cooling systems to patients with solid tumors undergoing chemotherapeutic protocols associated with a high risk of developing CIA, scalp cooling is not applicable to all chemotherapy regimens. For example, patients receiving platinum derivatives who develop severe peripheral neuropathies, which limit their tolerance to cold, should avoid scalp cooling [29]. Patients with hematological tumors should also avoid scalp cooling for their increased risk of scalp metastasis [9]. Furthermore, scalp cooling devices should be avoided for patients with cold agglutinin disease, cryoglobulinemia, and posttraumatic cold injury due to the risk of triggering local or generalized attack [30].
3. Topical Calcitriol
Vitamin D, partly synthesized within the keratinocytes in the presence of ultraviolet-B radiation and partly obtained from diet, plays an important role in dermatology and dermatotherapeutics due to its anti-inflammatory and immunomodulatory properties, as well as regulation of keratinocyte differentiation and proliferation [31]. Vitamin D is also believed to stimulate hair growth and anagen initiation, and its deficiency has been closely linked to various types of alopecia, including telogen effluvium, androgenetic alopecia, alopecia areata, trichotillomania, and scarring alopecia [31,32]. Although previous studies have failed to detect positive effects of calcitriol on patients with CIA—in fact, application of calcitriol (1,25-dihydroxyvitamin D3) caused a pruritic irritative dermatitis in five of the eight patients—further investigation of the drug was suggested because patients did not experience any systemic toxic effects [33]. Topical calcitriol was applied to 23 female breast and gynecologic cancer patients who were receiving a taxane-based chemotherapy, and a reduction of alopecia of >50% was observed in 8 patients at Week 7 (NCT01588522) (Table 2) [34]. The safe dosage was determined as 80 μg/mL [34].
4. Keratinocyte Growth Factor (KGF)
Keratinocyte growth factor (KGF), also called fibroblast growth factor 7 (FGF7), is a potent mitogen that regulates the migration and differentiation of various epithelial cells and protects them from various insults under stress conditions [35]. Intradermal injections of a bioengineered hair formulation containing various growth factors, including KGF, into the scalp, once every 3 weeks for a total of eight sessions, resulted in significant reduction in hair fall, decreased number of vellus hairs, and increased number of terminal hairs and shaft diameter [36]. In addition, in a neonatal rat model of cytosine arabinoside-induced alopecia, pretreatment with recombinant KGF alone induced a dose-dependent cytoprotective effect, abrogating as much as 50% of the alopecia [37]. Furthermore, in human scalp HF organ culture, KGF pretreatment slightly, but significantly, inhibited HF apoptosis and dystrophy induced by 4-hydroperoxycyclophosphamide (4-HC), a key cyclophosphamide metabolite [38]. A study to investigate KGF hair serum for the prevention of CIA (NCT04554732) was recently completed (Table 2).
5. Topical Vasoconstrictors
Topical vasoconstrictors have also been shown to be effective in protecting against CIA and oral mucositis, but only in animal models to date. In one study, when epinephrine was topically applied to the dorsal skin of neonatal rats, a 95% coat retention (suppression of alopecia) was observed in rats treated with N-nitroso-N-methylurea, and a 16% coat retention was observed in rats treated with systemic Cytoxan [39]. The promising result justifies further testing to determine whether vasoconstrictors can protect against CIA in humans. A major advantage of vasoconstrictors over scalp cooling is that they can be applied when necessary, while scalp cooling is only administered at the time of infusion. Because the half-life of chemotherapeutic drugs is longer than their infusion time, vasoconstriction through scalp cooling at the time of infusion cannot prevent their toxic effects in the subsequent days/weeks. Additionally, topical vasoconstrictors can be used for patients who are not good candidates for scalp cooling [9].
6. Low Intensity Ultrasound (LIUS)
Recently, low intensity ultrasound (LIUS) was shown to reverse the cytotoxic damage of paclitaxel on microtubules in cultured cells [40,41]. Given their excellent safety profile and availability as inexpensive home devices, it is important to explore the potential of LIUS application to prevent CIA.
7. Summary of Treatments and Supplementary Management
While prevention would be ideal, for patients who have already developed CIA, several agents, physical methods and injections shown to promote hair regrowth in other types of alopecia—such as alopecia areata and androgenetic alopecia—are being explored for their ability to also expedite hair regrowth after CIA. These include topical and oral minoxidil, photobiomodulation therapy (PBMT), platelet-rich plasma injections, and other oral and topical treatments.
Minoxidil is an antihypertensive vasodilator that also alters the temporal aspects of the hair cycle by extending the HF growth phase (anagen) while shortening the relative quiescent phase (telogen). While it is usually given topically, a low dosage of oral minoxidil has also been used in the treatment of pCIA, which is particularly useful for patients with poor compliance to topical minoxidil due to scalp irritation or hair texture [48]. Photobiomodulation therapy (PBMT), or low-level laser therapy, is widely used in medicine and dentistry because of its ability to accelerate healing by increasing cell viability [61,62]. Due to its excellent safety profile, non-invasive nature, relatively low costs, availability as home devices, and ease to us, PBMT is a popular option for patients and is used with high compliance rates [64]. PBMT has been shown to be effective in treating male and female pattern hair loss and alopecia areata [61,62,64,65], but its effectiveness in treating CIA needs to be further explored. Platelet-rich plasma (PRP) has been used widely in alopecia treatment because of its ability to induce cell proliferation, support HF growth, and extend anagen [72,79,80,81,82]. Additionally, spironolactone is a synthetic aldosterone receptor antagonist that has been used off-label for various dermatological conditions [85,86,87]. In a recent study, it has shown a moderate to significant improvement in hair regrowth in pCIA patients when combined with topical minoxidil [11].
Due to the tremendous psychological stress CIA can cause cancer patients, supplementary care and management are also important to explore in addition to treatment options. Patients experiencing negative psychological concerns should be advised to seek psychological support through therapy or patient associations or support groups [21,119]. For aesthetic purposes, the use of turbans, head scarfs, bandanas, and wigs can help improve patients’ QoL [21,120]. Additionally, camouflage techniques including cutting one’s hair shorter early on, keratin powder, and temporary tattooing also help [21,116,121,122]. When looking into the future, with the expected large increase in cancer survivors, it is important to find additional and more superior methods of palliative care that can help increase a patient’s QoL while dealing with and recovering from CIA.
8. Conclusions
The current approaches to prevent and treat CIA are summarized in Figure 1. Some of these devices/agents are being studied in clinical trials (Table 2), and positive results may bring new options for cancer patients facing chemotherapy. Presently, the FDA-cleared scalp cooling systems are the most effective strategy to prevent CIA for patients with solid tumors undergoing certain chemotherapy regimen. In addition, self-administered cold caps are also available from several manufacturers, although they are not cleared by the FDA. However, scalp cooling is not suitable for patients with hematological tumors or certain peripheral neuropathies, or for patients prone to cold injury, and costs and access remain an issue. Even in countries with universal healthcare systems, the extra time needed for the patient to stay in the chemotherapy room is a drawback. For those patients who cannot use scalp cooling, application of topical agents such as calcitriol, KGF, and vasoconstrictors may offer good alternatives. Topical agents also have the advantage of being applied as often as daily instead of only during infusion time for scalp cooling.
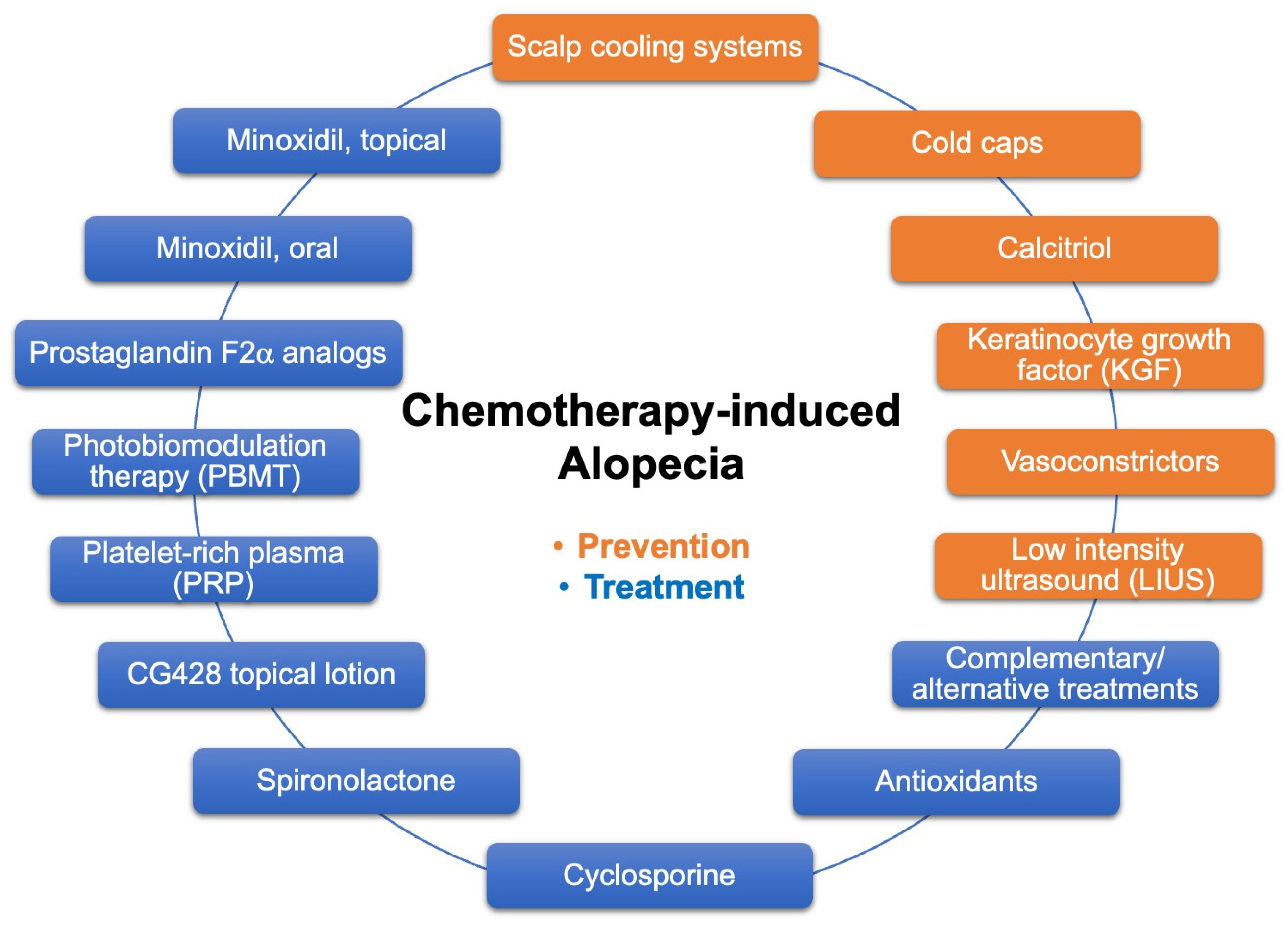
Figure 1. Current approaches to prevent and treat CIA.
In terms of treatment for CIA, the current options available include topical and oral minoxidil, PBMT, PRP injections, and other oral and topical treatments. Additionally, given the importance of scalp and facial hair in social communications of health, beauty, and youth, CIA has a significant negative impact on patients’ self-esteem, body image, sexuality, and overall QoL. Therefore, it is important to advise patients to seek psychological support and provide them with supplementary management methods such as wearing wigs, turbans, head scarfs, and using camouflage techniques.
Globally, new cancer cases are projected to reach 28.4 million in 2040 [123]. With prolonged life expectancy, earlier and more accurate diagnosis and more targeted treatment, the number of cancer survivors is expected to increase tremendously. In the U.S. alone, the number of cancer survivors is projected to grow from 18.1 million in 2022 to 26.0 million by 2040 [124]. Oncology teams who recognize the effects of CIA and work with dermatologists would better address CIA and provide better overall care to patients, and likely improve cancer treatment compliance and outcome.
Funding
T.C.W. is an Endowed Frost Investigator, Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine. This work is supported in part by AR079514 from NIH/NIAMS.
Conflicts of Interest
The authors declare no conflict of interest.
This entry is adapted from the peer-reviewed paper 10.3390/curroncol30040275
