Supercapacitors are energy storage devices with high power density, rapid charge/discharge rate, and excellent cycle stability. Carbon-based supercapacitors are increasingly attracting attention because of their large surface area and high porosity. Carbon-based materials research has been recently centered on biomass-based materials due to the rising need to maintain a sustainable environment. Cellulose and lignin constitute the major components of lignocellulose biomass. Since they are renewable, sustainable, and readily accessible, lignin and cellulose-based supercapacitors are economically viable and environmentally friendly.
- lignin
- cellulose
- nanofiber
- electrospinning
- supercapacitor
1. Introduction
Human activities, security, and industrialization depend on energy production and this energy is mostly derived from fossil fuels [1]. However, heavy consumption of fossil fuels poses a serious environmental challenge due to the emission of CO2, SO2, and NOx into the atmosphere [2]. The World Energy Council (www.worldenergy.org) has estimated that by 2050, the world would demand twice its present energy consumption. Thus, a green approach to an effective energy production system that can replace fossil fuels is necessary [2,3,4]. Recently, flexible supercapacitors are among the most investigated energy storage devices due to their high-power density, rapid charge/discharge rate, excellent cycle stability, and robust nature. The electrochemical double-layer capacitor (EDLC) is a specific type of energy storage system where the capacitance is taken from electrolyte adsorption and alignment at the interface between the electrolyte and a conductive porous electrode with a wide surface area [5]. The efficiency of the EDLC is determined by the properties of its chosen electrode (active) materials. Electrospun carbonaceous materials are used as electrode materials, conducting additives, and supporting substrates in EDLCs due to their excellent electrical conductivity, large surface area, high porosity, mechanical flexibility, and chemical stability [6]. Due to its wide surface area and high porosity, carbon-based supercapacitors draw more and more interest. Recently, carbon-based materials research focuses on biomass materials because of the growing need for sustainable environmental protection. Owing to its high melting point, rich carbon content, and quick speed in pyrolysis, polyacrylonitrile (PAN) is the most suitable precursor for manufacturing high-performance carbon fibers (CFs) as compared to other common precursors (such as polyaniline, pitch, rayon, etc.). PAN precursor electrospinning accompanied by stabilization and carbonization can be used for producing carbon nanofibers (CNFs) having finer and more regulated fiber diameters [7]. However, PAN and other petroleum-based polymers are quite expensive [4]. Additionally, PAN, polybenzimidazole, or pitch, as carbon sources release toxic substances during carbonization [8]. It is therefore necessary to opt for greener carbon sources for the production of CNFs.
Biomass cellulose (mostly from cellulose acetate, ethyl cellulose, methyl cellulose, etc.), being the most abundant biological macromolecules in nature, is a good precursor material for electrospinning CNFs due to its good flexibility and essential properties [9]. In the last few decades, CNFs has been fascinating, due to its abundance and low cost, while at the same time having special characteristics such as large surface area and high carbon content [10]. Cellulose consists of carbon, hydrogen, and oxygen components, and its carbonization mainly releases carbon dioxide and water. Cellulose does not have an apparent melting point and after successful carbonization, it preserves its physical morphology [8]. Compared to cellulose, it is easier to electrospin cellulose acetate into nanofibers and films because the solubility of cellulose acetate depends on the degree to which the acetate group is replaced [11]. However, cellulose is a macromolecular polysaccharide, a large number of oxygen atoms in cellulose structure are highly autoxidized at high temperatures, thereby causing thermal instability. This thermal instability leads to morphology collapse during carbonization [9]. The second most abundant bio-based polymer is lignin. It is obtained in large quantities from the pulp, paper, and bio-ethanol industries as a coproduct [12]. Lignin is a rich carbon source due to its many aromatic subunits [13]. Electrospun lignin-based CNFs have large surface area, excellent graphitic and porous structures, and superior chemical stability against corrosive elements [14]. However, the structural units in lignin are non-linear and are randomly interconnected by numerous C-C and ether bonds, which together help to make the lignin polymer poorly flexible [15]. Thus, clean lignin materials are comparatively delicate and cannot be easily manipulated. Furthermore, the randomly distributed aliphatic chains separating the aromatic units in lignin reduces its tensile strength and moduli dramatically during thermal treatment [16]. Diversity of lignin biopolymers is another serious problem affecting batch reproducibility of lignin derived CNFs [16]. Nevertheless, lignin may be mixed with plasticizers in order to obtain good fibers [13].
The study into CNFs made from a lignin/cellulose blend is a new research field that needs to be explored due to the rising need for energy storage devices based on biomass CNFs. As far as we know, only a few papers on electrospun lignin/cellulose nanofibers for energy storage have been published. Interestingly, the few studies show that supercapacitors based on lignin/cellulose may be an exotic area of research interest in the field of supercapacitors based on carbon biomass precursors. Besides, both lignin and cellulose are green and sustainable, and their extraction methods are very simple, economical, and eco-friendly. The need to investigate this new field is, therefore, very evident. Many reviews on electrospun lignin and cellulose nanofibers have been published. However, very few systemic reports are available on both lignin and cellulose nanofibers. In particular, no review of electrospun nanofibers from a blend of these abundant biomass materials has been published.
2. Studies on Electrospun Lignin and Cellulose Nanofibers
2.1. Studies on Electrospun Cellulose Nanofibers
Cellulose is the most abundant natural macromolecule and the main ingredient of lignocellulose biomass. It has a high carbon content of around 44%, high stability, and outstanding porosity due to its hierarchical composition and highly functional rigid linear chains [22,23]. Cellulose is well known as a precursor material for the processing of CNFs. In the late 60 s rayon (a fabric made from cellulose) based fibers became the first commercial carbon fibers [24]. Because cellulose does not dissolve in many organic solvents, cellulose nanofibers are prepared from different derivatives of cellulose such as cellulose acetate (CA) and ethyl cellulose (EC) [8]. In this study, 13 articles on electrospun cellulose nanofibers meet our inclusion criteria. Therefore, this section presents a review of these 13 articles on electrospun cellulose nanofibers.
Precursor Solution for Electrospun Cellulose Nanofibers
In CNF formation, the selection of an appropriate solvent is extremely significant. The right solvent that can evaporate quickly during the electrospinning process is required to facilitate jet elongation leading to nanofiber formation. Cellulose electrospinning solution is difficult to prepare because it is normally not soluble in most organic solvents. However, cellulose acetate (CA) is much easier to electrospin because the solubility of CA depends on the degree to which acetate group is replaced. Therefore, CA is the most widely used cellulose derivative to prepare cellulose nanofibers. According to one report, CA was dissolved in acetone and dimethyl acetamide (DMAc) to electrospin CA nanofibers followed by hydrolysis in NaOH/ethanol solution at room temperature for 12 h prior to thermal treatment [25]. These nanofibers were easily electrospun because the mixture of DMAc and ethanol provided ample surface tension to interplay with the viscoelastic force, thereby forming the Taylor cone. As can be seen in SEM images in Figure 8, the resultant nanofibers show a melting phenomenon due to morphological collapse. The morphological collapse could be due to short soaking time in DMAc/ethanol solution leading to incomplete deacetylation. Formation of higher fibrous morphology could be attributed to increasing ZnCl2 content in the precursor solution. Ths reveals that ZnCl2 increases thermal and structural stability by efficient ion interlinking of CA macromolecules, thus promoting dehydrogenation.
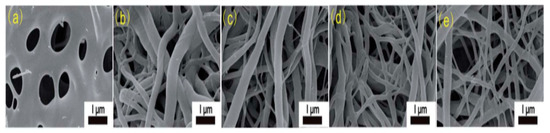
Figure 8. SEM images of electrospun cellulose acetate nanofibers (dissolved in acetone/DMAc) at different ZnCl2 content (a) CACNF-0, (b) CACNF-ZnCl2-2%, (c) CACNF-ZnCl2-5%, (d) CACNF-ZnCl2-10%, (e) CACNF-ZnCl2-20% [25].
2.2. Studies on Electrospun Lignin Nanofibers
Lignin is the second most abundant natural bioresource material, after cellulose. It provides structural supports to plants because of its rigid nature. According to the International Lignin Institute, there are approximately 300 billion metric tons of lignin on earth with an annual estimated production of 40 to 50 million tons as by-products of the pulp and paper industries and biorefineries. Lignin has a high carbon content, almost 60–65%, which indicates a possible high yield following carbon fiber processing [51]. Carbon fibers from lignin precursor material were first reported by Kubo et al. [52]. The authors proposed the conversion of lignin into functional polymers by an efficient separation procedure to extract the spinnable material from lignin. After thermal stabilization, the prepared fibers were carbonized to convert lignin fibers to carbon fibers. Currently, electrospun lignin nanofibers are prepared either by physically blending lignin with other polymers or by chemically modifying lignin structure [36,42,53,54,55]. This section will analyze those articles that investigated electrospun lignin nanofibers.
Chemical Modification of Lignin Structure
Foreign elements can be introduced into lignin precursor solution to alter the non-linear and randomly entangled numerous C-C and ether bonds which makes lignin rigid and difficult to electrospin. Using a simple heated single spinneret system, Tunnapat and Surawut [36] were able to electrospin water-soluble lignin nanofiber by adding glycerol into the precursor solution. Addition of glycerol reduced surface tension of the precursor solution thereby improving the spinnability of lignin. At a higher glycerol ratio, the fiber diameter and BET surface area increase while electrical conductivity decreases. Decreased fiber diameter can be due to glycerol evaporation during thermal stabilization whereas decreased electrical conductivity may mean that glycerol plays a major role in altering the lignin structure and improving spinnability. Similarly, increased surface area at higher glycerol ratio can be attributed to successive evaporation of glycerol during thermal stabilization, reducing the resulting average fiber diameter. In another report, lignin/H3PO4 nanofibers were prepared via coaxial electrospinning technique of Alcell lignin in ethanol (core spinning solution) and pure ethanol (shell spinning solution) [12]. Electrospinning of lignin nanofiber was assisted by the presence of H3PO4 in the precursor solution. H3PO4 could effectively dehydrate lignin phenolic-OH group to form phospholipid bonds between lignin molecules, thereby increasing the spinnability of lignin [56]. Lignin nanofibers were also prepared by adding isophorone diisocyanate (IPDI) to the spinning solution [57]. IPDI could link adjacent lignin molecules by establishing stable covalent bonds between them. SEM images (not shown) of lignin nanofibers prepared by either phosphating process or by covalent bonding neither have beads nor show evidence of morphological collapse after carbonization. Evidently, stable lignin fibers were formed because of continuous dehydration of phenolic hydroxyl groups in lignin by H3PO4 and IPDI, respectively.
2.3. Studies on Electrospun Lignin/Cellulose Nanofibers
The fascinating characteristics of lignin and cellulose nanofibers such as low cost, high carbon performance, wide-field, mechanical stability, and environmental sustainability make them promising candidates for carbon-based EDLC electrode materials. Lignin and cellulose nanofibers can be combined into lignin/cellulose nanofibers to optimize the advantages of lignin and cellulose biomass materials as energy storage devices [54]. Physical blending of lignin and cellulose generally involves modifying the -OH groups phenolic group of lignin and cellulosic material (acetyl group in the case of cellulose acetate) to allow crosslinking reaction between lignin and cellulose macromolecules [36]. To the best of our knowledge, only eight studies on carbon nanofibers derived from the physical blending of electrospun lignin and cellulose precursors were reported.
Lignin/Cellulose Acetate (CA) Blend
Lignin/CA nanofibers were obtained from continuous dehydration of phenolic hydroxyl group of lignin and cellulosic hydroxyl group to create electron-deficient oxygen atoms. These oxygen atoms can successfully combine with electron-rich atoms from binding agents to form stable bonds between lignin and cellulose acetate [53]. Early attempts to prepare lignin/cellulose fibers met with little success due to the numerous formations of beaded fibers and subsequent collapse of fiber morphology. In 1997, Dave and Glasser reported that adding lignin to cellulose acetate butyrate disrupted the nanocrystalline order of the cellulosic phase [80]. Similarly, electrospun lignin/CA nanofibers with smooth and defect-free morphology were reported [81]. However, fibrous morphologies of these nanofibers were completely lost after carbonization due to phase separation between lignin and cellulose macromolecules. The SEM images of the prepared nanofibers after and before carbonization are shown in Figure 12. Loss of fibrous morphology after carbonization was obviously due to weak interaction between lignin phenolic group and cellulose acetyl group resulting in phase separation.
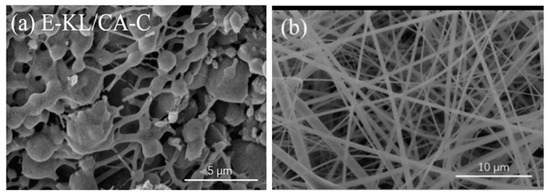
Figure 12. SEM images of electrospun lignin cellulose acetate nanofibers (a) after carbonization and (b) before carbonization. Adapted with permission from ref [81].
3. Conclusions
Biomass carbon nanofiber technology is one of the most active research areas in energy storage devices, especially EDLCs. The main objective of this systematic review is to highlight the state of the art research on electrospun lignin and cellulose nanofibers for application in supercapacitors and to provide directions of future research on the area. A rigorous scientific approach was employed to screen the eligibility of included articles and taxonomy of literature was provided from the most relevant articles included in the literature review. Apart from the articles in the taxonomy of literature, nine other articles were also included in our critical review and development of the research framework. These articles did not strictly meet our inclusion criteria, but they contain vital information that helped in developing the research framework. From our included literature, four main categories were discussed: reviews, studies on electrospun lignin nanofibers, cellulose nanofibers, and lignin/cellulose nanofibers. While studies on electrospun lignin and cellulose-based supercapacitors are considered adequate, very limited studies were reported on lignin/cellulose based supercapacitors. However, the reported studies on lignin/cellulose-based supercapacitors indicate a new research direction for biomass-based supercapacitors. From the articles reviewed in this study, researchers stated some major challenges related to large scale application of these special class of electrode materials. In the same vein, recommendations were given to mitigate these challenges. Specifically, comments and suggestions include:
-
Electrospun lignin and cellulose nanofiber-based electrodes have a wide surface area, good porosity, mechanical stability, and excellent cycle stability. However, the energy density of these electrode materials is quite low compared to other carbon-based electrode materials like PAN, graphene, etc. Similarly, carbon nanofibers produced from cellulosic precursors undergo morphological collapse during carbonization due to the low thermal stability of cellulose derivatives. Deacetylation of CA prior to thermal treatment improves the thermal stability of CA-based carbon nanofibers.
-
Electrospinning lignin into nanofibers is unrealistic due to the hierarchical and entangled structure of lignin. However, lignin can be easily blended with other binders to produce fine lignin-based nanofibers. Different binders influence electrospun lignin nanofiber differently.
-
Electrospinning lignin and cellulose precursor solution resulted in nanofiber with phases separated into lignin and cellulose domains. However, incorporating a suitable crosslinker resolves the problem of phase separation. Resultant nanofibers exhibit thermal stability of lignin and flexibility of CA.
-
Lignin/cellulose nanofiber, if explored extensively, can serve as ideal electrode materials for next-generation supercapacitors as an alternative to petroleum-based carbon nanofibers
