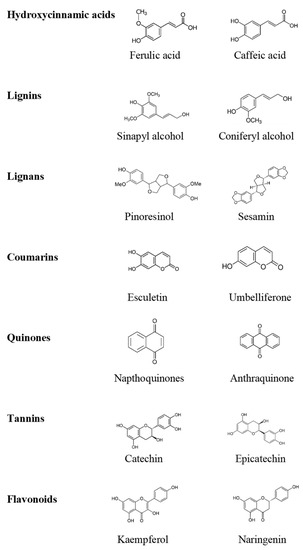
2. Different Types of Phenolic Compounds and Their Biosynthesis
Plants produce an exceptionally diverse array of low molecular mass compounds, often called secondary metabolites, and they are essential for anticipating and responding to biotic and abiotic stress. [
6]. These metabolites are generally derived from different biosynthetic routes such as isoprenoid, phenylpropanoid, alkaloid, or fatty acid pathways. The phenolic compound is derived either from the shikimic acid pathway or pentose phosphate through phenylpropanoid metabolism [
7]. The phenolic compound contains benzene rings with one or more hydroxyl substituents, which are synthesized from simple phenolic molecules to highly polymerized compounds according to their demand function. The primary metabolisms, such as glycolysis and pentose phosphate pathways, provide compounds such as phosphoenolpyruvate and erythrose-4-phosphate to initiate the biosynthesis of phenolic compounds. Together, phosphoenolpyruvate and erythrose-4-phosphate produce shikimic acid via the shikimate pathway. Further, this pathway provides L-phenylalanine, which takes part in the phenylpropanoid pathway to produce
p-coumaroyl CoA. This compound initiates the synthesis of phenylpropanoids and monolignols to produce more complex phenolic compounds such as stilbenes or flavonoids after reacting with three malonic acid molecules [
8] (
Figure 2).
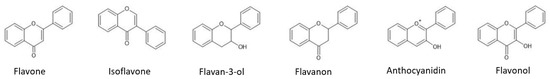
Figure 2. Structural representation of the major flavonoid classes.
2.1. Flavonoids
Flavonoids are polyphenolic molecules containing 15 carbon atoms with two aromatics rings. Flavonoids are the most diverse class of phenolic compounds and are present in all types of plants within the plant kingdom [
9]. They mostly accumulate in very high concentrations in the leaf’s epidermis and fruits’ skins. The flavonoid biosynthesis is mainly initiated through the phenylpropanoid pathway. During normal conditions, flavonoids act as a signaling molecule and UV protectant and regulate plant hormones such as auxin and cytokinin [
10]. However, during stress conditions, they protect from oxidative damage [
11]. Stress-induced dihydroxy B-ring-substituted flavonoids actively scavenge ROS [
12]. Flavonoids are classified into many groups, and the main subclasses are flavonols, flavan-3-ols, isoflavones, flavones, anthocyanidins, and flavanones (
Figure 2). Minor flavonoid groups are dihydroflavonols, flavan-3,4-diols, coumarins, chalcones, dihydrochalcones, and aurones. Flavonoids contain various substituents such as hydroxyl groups, present at the skeleton’s 4th, 5th, and 7th positions.
2.1.1. Flavonoid Biosynthetic Pathway
Plants produce an exceptionally diverse array of low molecular mass compounds, often called secondary metabolites, which are essential for anticipating and responding to biotic and abiotic stress. These metabolites are generally derived from different biosynthetic routes such as isoprenoid, phenylpropanoid, alkaloid, or fatty acid pathways. The phenolic compound is derived either from the shikimic acid pathway, phenylpropanoid, or both [
13]. The shikimic acid pathway is a major route for the biosynthesis of aromatic compounds in plants and microorganisms. Phenylalanine, tyrosine, and tryptophan are the primary metabolites that provide the precursor for most of the secondary metabolite compounds. Flavonoids are synthesized from phenylalanine which is derived from the shikimate pathway [
7,
14] (
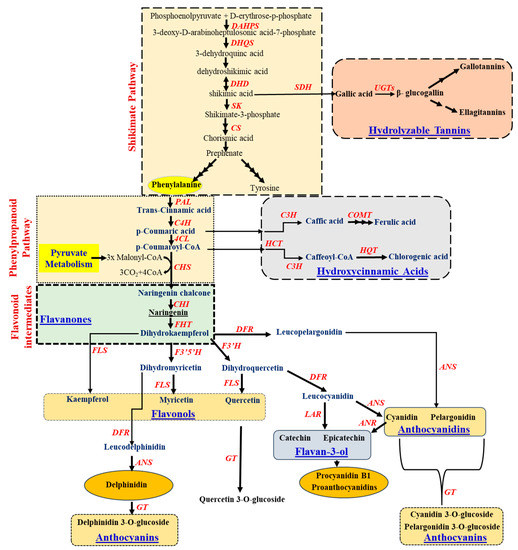
Figure 3). The shikimate pathway initiates with the molecule as phosphoenol pyruvate (PEP) and D-erythrose-p-phosphate and forms the intermediate precursor-shikimic acid following several steps (
Figure 3). This shikimic acid gets converted to chorismic acid, which leads to the synthesis of phenylpropanoid pathway, starts with aromatic amino acid, phenylalanine, and tyrosine. The derived phenylalanine gets converted to coumaric, caffeic, and ferulic acids through the activity of phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumarate: CoA ligase (4CL), which ultimately opens the lignin biosynthesis route in the plant systems. The coumaric acid also leads towards the biosynthesis of hydroxycinnamic acids such as caffeic, ferulic, and chlorogenic acids. On the other hand, the shikimic acid from the shikimate pathway also contributes towards the synthesis of gallic acid by the action of shikimate dehydrogenase (SDH). Synthesized gallic acid is then converted to glucogallin by UDP-3-glucosyltransferase (UGT), which then produces two important tannins-gallotannins and ellagitannins of the hydrolysable tannin pathway (
Figure 3).
Figure 3. Schematic representation of the flavonoids and their intermediates (shikimate, phenylpropanoid, flavanones, flavanols, flavan-3-ol, flavanol glycosides, anthocyanidins and anthocyanin biosynthetic pathway). The respective enzymes catalyzing the reaction in each pathway have been denoted in red colour. Abbreviation: 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthase (DAHPS); 3-dehydroquinate synthase (DHQS); 3-dehydroquinate dehydratase (DHD); Shikimate kinase (SK); chorismate synthase (CS); shikimate dehydrogenase (SDH), UDP-3-glucosyltransferase (UGT); Phenylalanine ammonia lyase (PAL); cinnamate-4-hydroxylase (C4H); 4-coumarate-CoA ligase (4CL); coumaryol-3-hydroxylase (C3H); Caffeoyl-O-methyltransferase (COMT); Chalcone synthase (CHS); Hydroxycinnamoyl-CoA: skimimate/quinate hydroxycinnamoyltransferase (HCT); hydroxycinnamoyl (CoA); quinate hydroxycinnamoyl transferase (HQT); chalcone-flavanone isomerase (CHI); fatty alcohol hydroxycinnamoyl transferase (FHT); flavonoid 3′,5′-hydroxylase (F3′5′H); flavonol 3′ hydroxylase (F3′H); dihydroflavonol 4-reductase (DFR); flavonol synthase (FLS); anthocyanidin synthase (ANS); anthocyanidin reductase (ANR); leucocyanidin reductase (LAR); flavonoid-3-O-glucosyltransferase (GT).
In general, the flavonoid biosynthesis pathway (Figure 3) also includes several intermediates such as flavanones (naringenin, hesperitin, dihydrokaempferol and eriodictyol), flavanols (kaempferol, myricetin, quercetin) and flavanone glycosides (delphinidin 3-O-glucoside, quercetin 3-O-glucoside). Flavanones are an important phenolic class that mostly occurs in citrus fruits such as lemons and oranges. The formation of naringenin chalcone from the condensation of p-coumaroyl-CoA with three malonyl CoA residues, catalyzed by chalcone synthase (CHS), is described in Figure 3. The product naringenin chalcone is then converted to naringenin by the action of chalcone isomerase (CHI) and finally to dihydrokaempferol. This dihydrokaempferol further diverges for the production of different classes of flavonoids, including isoflavones, flavanones, flavones, flavanols, flavan-3-ols and anthocyanins (Figure 3).
2.1.2. Flavone and Flavanone Biosynthesis
The dehydrogenation of flavanones results in the formation of flavones (2-aryl-4H-chromen-4-ones) by the action of enzyme flavone synthase via the conversion of flavanones to flavones, for example as leteolin, apigenin, and galangin. Such conversion requires NADPH and oxygen to introduce a double bond between the C-2 and C-3 residues in flavanones (Figure 3).
2.1.3. Isoflavonoid Biosynthesis
A leguminous plant predominantly accumulates the isoflavonoids and the responsible enzymes for their biosynthesis, as have been identified and characterized earlier [
15]. During these pathways, microsomal cytochrome P450 isoflavone synthase catalyzes and converts naringenin and isoquiritigenin into the isoflavones genistein and daidzein, respectively [
16].
2.1.4. Anthocyanin Biosynthesis
Cyanidin, pelargonidin, and delphinidin are the most commonly explored anthocyanidins; they are present in flowers and fruits and are also responsible for their distinct colour variations [
13]. The regulatory mechanism of anthocyanin biosynthesis has been explored broadly, starting with the hydroxylation of flavanones to produce dihydroflavonols by flavanone 3-hydroxylase (F3H) (
Figure 3). Then, dihydroflavonols were reduced to flavan-3,4-diols (leucoanthocyanins) by the enzyme dihydroflavonol reductase (DFR). Anthocyanidin synthase (ANS) catalyzes the last step in the biosynthesis of anthocyanins. Finally, Flavonoid-3-
O-glucosyltransferase (FGT) transfers the glucosyl moiety from UDP glucose to the 3-hydroxyl group of anthocyanidins and forms anthocyanins (
Figure 3).
2.2. Non-Flavonoids
Non-flavonoids are classified as phenolics acid, tannins, stilbenes and lignans [
17]. Most of the non-flavonoids present in fruits and vegetables have dietary significance. Phenolic acids such as gallic acid, acts as a precursor of hydrolysable tannins. Similarly, C6–C3 hydroxycinammates (p-Coumaroyl) and their derivatives are polyphenolic C6–C2–C6 stilbenes. However, lignans derived from phenylpropanoid E-coniferyl alcohol.
Stilbene Biosynthesis
The stilbene is also synthesized by the condensation of p-coumaroyl CoA with three units of malonyl CoA, catalyzed by the enzyme stilbene synthase. Stilbene synthase and chalcone synthase (CHS) are structurally very similar enzymes. Stilbene synthase is induced by a wide range of stresses such as UV radiation and bacterial and fungal infections [
17].