Consuming fresh food is undoubtedly the best way to enjoy various flavors and nutrients, but their preservation helps to enjoy all these even out of season. Bio-coating technologies hold great promise for the future of food preservation, offering a more sustainable and healthy way to keep fruits and vegetables fresh for more extended periods. The choice of a coating method may depend on the type of fresh fruits and vegetables, the coating material, and the desired coating thickness. The application method should be carried out under hygienic conditions to prevent contamination and ensure the effectiveness of the coating. It is also essential to apply the coating evenly and that it adheres properly to the surface of the produce, maximizing its effectiveness. The coating material can be applied in its pure form or mixed with other ingredients such as antioxidants, preservatives, or antimicrobial agents, thus enhancing its effectiveness.
- food safety
- bio-coatings
1. Introduction
2. Bio-Coatings Methods for Fruits and Vegetables Preservation
2.1. Methods to Prepare the Bio-Coatings
2.1.1. Nanoencapsulation
2.1.2. Microemulsion Formulation
2.1.3. Microspinning (Electrospinning)
2.1.4. Melt Extrusion
2.1.5. Coacervation
2.1.6. Phase Inversion Method
2.2. Methods of Coating Application in Fresh Fruits and Vegetables
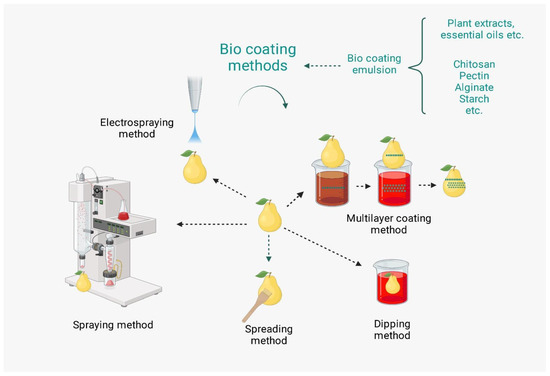
2.2.1. Dipping (Dip Coating) Method
2.2.2. Spraying Method
2.2.3. Spreading Method
2.2.4. Vacuum Infusion
2.2.5. Solution Casting Process
2.2.6. Multilayer Coating (Layer by Layer)
2.2.7. Cross-Linked Coating Method
2.2.8. D Food Printing Method
This entry is adapted from the peer-reviewed paper 10.3390/coatings13081420
References
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2021, 19, 1715–1735.
- Devi, M.P.; Bhowmick, N.; Bhanusree, M.R.; Ghosh, S.K. Preparation of Value-Added Products Through Preservation. In Value Addition of Horticultural Crops: Recent Trends and Future Directions; Sharangi, A.B., Datta, S., Eds.; Springer India: New Delhi, India, 2015; pp. 13–41.
- Jaya Shankar, T. Introductory Chapter: Food Processing, Preservation, and Packaging—A Brief Overview. In Food Processing and Packaging Technologies; Jaya Shankar, T., Ed.; IntechOpen: Rijeka, Croatia, 2023; Chapter 1.
- Cocetta, G.; Natalini, A. Ethylene: Management and breeding for postharvest quality in vegetable crops. A review. Front. Plant Sci. 2022, 13, 968315.
- Factors Affecting Ripening. Available online: http://eagri.org/eagri50/HORT381/lec04.html (accessed on 14 July 2023).
- Basic Agricultural Study—A Resource Hub for Young Agriculturists. Available online: https://agriculturistmusa.com/maturity-indices-types-and-determination/?utm_content=cmp-true (accessed on 15 July 2023).
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119.
- Maduwanthi, S.D.T.; Marapana, R. Induced Ripening Agents and Their Effect on Fruit Quality of Banana. Int. J. Food Sci. 2019, 2019, 2520179.
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging Postharvest Technologies to Enhance the Shelf-Life of Fruit and Vegetables: An Overview. Foods 2022, 11, 3925.
- Lufu, R.; Ambaw, A.; Opara, U.L. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020, 272, 109519.
- Strategies to Reduce Post-Harvest Losses for Fruits and Vegetables. Available online: http://www.postharvestproject.com/uploads/outputs/8fa991f1-6260-45e4-95b0-438127a4deb0.pdf (accessed on 14 July 2023).
- Odetayo, T.; Tesfay, S.; Ngobese, N.Z. Nanotechnology-enhanced edible coating application on climacteric fruits. Food Sci. Nutr. 2022, 10, 2149–2167.
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68.
- Muhammad Sajid, A.; Syeda Ayesha, B. Natural Antimicrobials, their Sources and Food Safety. In Food Additives; Desiree Nedra, K., Geethi, P., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 4.
- Chung, I.; Ryu, H.; Yoon, S.Y.; Ha, J.C. Health effects of sodium hypochlorite: Review of published case reports. Environ. Anal. Health Toxicol. 2022, 37, e2022006.
- Wu, P.H.; Chang, H.X.; Shen, Y.M. Effects of synthetic and environmentally friendly fungicides on powdery mildew management and the phyllosphere microbiome of cucumber. PLoS ONE 2023, 18, e0282809.
- Kori, R.K.; Singh, M.K.; Jain, A.K.; Yadav, R.S. Neurochemical and Behavioral Dysfunctions in Pesticide Exposed Farm Workers: A Clinical Outcome. Indian J. Clin. Biochem. 2018, 33, 372–381.
- Sun, S.H.; Kim, S.J.; Kwak, S.J.; Yoon, K.S. Efficacy of sodium hypochlorite and acidified sodium chlorite in preventing browning and microbial growth on fresh-cut produce. Prev. Nutr. Food Sci. 2012, 17, 210–216.
- Raffo, A.; Paoletti, F. Fresh-Cut Vegetables Processing: Environmental Sustainability and Food Safety Issues in a Comprehensive Perspective. Front. Sustain. Food Syst. 2022, 5, 681459.
- Gadelha, J.R.; Allende, A.; López-Gálvez, F.; Fernández, P.; Gil, M.I.; Egea, J.A. Chemical risks associated with ready-to-eat vegetables: Quantitative analysis to estimate formation and/or accumulation of disinfection byproducts during washing. EFSA J. 2019, 17, e170913.
- Magami, S. Functional can coatings—Part 2: Composition, attributes, applications and performance. Surf. Coat. Int. 2015, 96, 148–155.
- Das, S.; Ghosh, A.; Mukherjee, A. Nanoencapsulation-Based Edible Coating of Essential Oils as a Novel Green Strategy against Fungal Spoilage, Mycotoxin Contamination, and Quality Deterioration of Stored Fruits: An Overview. Front. Microbiol. 2021, 12, 768414.
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215.
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in Nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042.
- de Oliveira Filho, J.G.; Miranda, M.; Ferreira, M.D.; Plotto, A. Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation. Foods 2021, 10, 2438.
- Tripathi, A.D.; Sharma, R.; Agarwal, A.; Haleem, D.R. Nanoemulsions based edible coatings with potential food applications. Int. J. Biobased Plast. 2021, 3, 112–125.
- Horison, R.; Sulaiman, F.O.; Alfredo, D.; Wardana, A. Physical characteristics of nanoemulsion from chitosan/nutmeg seed oil and evaluation of its coating against microbial growth on strawberry. Food Res. 2019, 3, 821–827.
- Sessa, M.; Ferrari, G.; Donsì, F. Novel Edible Coating Containing Essential Oil Nanoemulsions to Prolong the Shelf Life of Vegetable Products. Chem. Eng. Trans. 2015, 43, 55–60.
- Kim, I.-H.; Lee, H.; Kim, J.E.; Song, K.B.; Lee, Y.S.; Chung, D.S.; Min, S.C. Plum Coatings of Lemongrass Oil-incorporating Carnauba Wax-based Nanoemulsion. J. Food Sci. 2013, 78, E1551–E1559.
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 2020, 10, 1657.
- Paul, B.K.; Moulik, S.P. Uses and applications of microemulsions. Curr. Sci. 2001, 80, 990–1001.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415.
- Shi, C.; Fang, D.; Huang, C.; Lyu, L.; Wu, W.; Li, W. Electrospun biopolymer material for antimicrobial function of fresh fruit and vegetables: Application perspective and challenges. LWT 2023, 174, 114374.
- Gagaoua, M.; Pinto, V.Z.; Göksen, G.; Alessandroni, L.; Lamri, M.; Dib, A.L.; Boukid, F. Electrospinning as a Promising Process to Preserve the Quality and Safety of Meat and Meat Products. Coatings 2022, 12, 644.
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and Characterization of Electrospun Food Biopackaging Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Derived from Fruit Pulp Biowaste. Front. in Sustain. Food Syst. 2018, 2, 38.
- Merino, D.; Quilez-Molina, A.I.; Perotto, G.; Bassani, A.; Spigno, G.; Athanassiou, A. A second life for fruit and vegetable waste: A review on bioplastic films and coatings for potential food protection applications. Green Chem. 2022, 24, 4703–4727.
- Tavares, L.; Souza, H.K.S.; Gonçalves, M.P.; Rocha, C.M.R. Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate. Food Hydrocoll. 2021, 113, 106471.
- Ramos, M.; Mellinas, C.; Solaberrieta, I.; Garrigós, M.C.; Jiménez, A. Emulsions Incorporated in Polysaccharide-Based Active Coatings for Fresh and Minimally Processed Vegetables. Foods 2021, 10, 665.
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering 2023, 5, 520–536.
- Jose, A.; Pareek, S.; Radhakrishnan, E.K. Advances in Edible Fruit Coating Materials. In Advances in Agri-Food Biotechnology; Sharma, T.R., Deshmukh, R., Sonah, H., Eds.; Springer Singapore: Singapore, 2020; pp. 391–408.
- Pirozzi, A.; Ferrari, G.; Donsì, F. The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables. Coatings 2021, 11, 990.
- Giray Tufan, E.; Akpinar Borazan, A.; Koçkar, Ö.M. A Review on Edible Film and Coating Applications for Fresh and Dried Fruits and Vegetables. BSEU J. Sci. 2021, 8, 1073–1085.
- Moncayo-Martínez, D.; Buitrago, G.; Enciso, N. The surface properties of biopolymer-coated fruit: A review. Ing. Investig. 2013, 33, 11–16.
- Ghosh, M.; Singh, A.K. Potential of engineered nanostructured biopolymer based coatings for perishable fruits with Coronavirus safety perspectives. Prog. Org. Coat. 2022, 163, 106632.
- Shiekh, K.A.; Ngiwngam, K.; Tongdeesoontorn, W. Polysaccharide-Based Active Coatings Incorporated with Bioactive Compounds for Reducing Postharvest Losses of Fresh Fruits. Coatings 2022, 12, 8.
- Senturk Parreidt, T.; Schmid, M.; Müller, K. Effect of Dipping and Vacuum Impregnation Coating Techniques with Alginate Based Coating on Physical Quality Parameters of Cantaloupe Melon. J. Food Sci. 2018, 83, 929–936.
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable by-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857.
- Aphirak, P.; Hathaitip, R.; Tri Indrarini, W. Effect of Fruit Size and Processing Time on Vacuum Impregnation Parameters of Cantaloupe and Apple. CMU J. Nat. Sci. 2015, 14, 125–132.
- Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Kidoń, M. Applicability of Vacuum Impregnation to Modify Physico-Chemical, Sensory and Nutritive Characteristics of Plant Origin Products—A Review. Int. J. Mol. Sci. 2014, 15, 16577–16610.
- Neegam, N.; Gunjan, K.K.; Sawinder, K.; Prasad, R. Recent Developments in Edible Coatings for Fresh Fruits and Vegetables. J. Hortic. Res. 2021, 29, 127–140.
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024.
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-based functional films for packaging applications: A review. Front. Nutr. 2022, 9, 1000116.
- Moeini, A.; Pedram, P.; Fattahi, E.; Cerruti, P.; Santagata, G. Edible Polymers and Secondary Bioactive Compounds for Food Packaging Applications: Antimicrobial, Mechanical, and Gas Barrier Properties. Polymers 2022, 14, 2395.
- Rossi-Márquez, G.; Dávalos-Saucedo, C.A.; Mayek-Pérez, N.; Di Pierro, P. Multilayered Edible Coatings to Enhance Some Quality Attributes of Ready-to-Eat Cherimoya (Annona cherimola). Coatings 2023, 13, 41.
- Arnon-Rips, H.; Poverenov, E. Improving food products’ quality and storability by using Layer by Layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81–92.
- Andriani, V.; Abyor Handayani, N. Recent technology of edible coating production: A review. Mater. Today Proc. 2023, in press.
- Gutiérrez-Jara, C.; Bilbao-Sainz, C.; McHugh, T.; Chiou, B.-S.; Williams, T.; Villalobos-Carvajal, R. Effect of Cross-Linked Alginate/Oil Nanoemulsion Coating on Cracking and Quality Parameters of Sweet Cherries. Foods 2021, 10, 449.
- Tihan, G.T.; Zgarian, R.G.; Berteanu, E.; Ionita, D.; Totea, G.; Iordachel, C.; Tatia, R.; Prodana, M.; Demetrescu, I. Alkaline Phosphatase Immobilization on New Chitosan Membranes with Mg2+ for Biomedical Applications. Mar. Drugs 2018, 16, 287.
- Cheng, Y.; Fu, Y.; Ma, L.; Yap, P.L.; Losic, D.; Wang, H.; Zhang, Y. Rheology of edible food inks from 2D/3D/4D printing, and its role in future 5D/6D printing. Food Hydrocoll. 2022, 132, 107855.
- Qiu, L.; Zhang, M.; Bhandari, B.; Chitrakar, B.; Chang, L. Investigation of 3D printing of apple and edible rose blends as a dysphagia food. Food Hydrocoll. 2023, 135, 108184.
